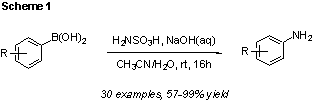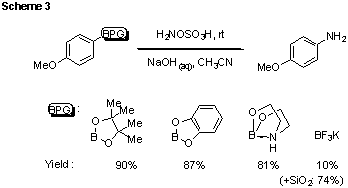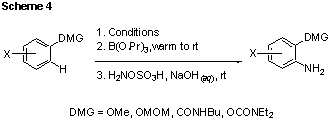Reports: UR152231-UR1: Boron-Based Directing Groups for Directed Lithiation Reactions
J. Adam McCubbin, PhD, University of Winnipeg
Through initial optimization studies we identified hydroxylamine-O-sulfonic acid (HSA) as a suitable, inexpensive and widely available aminating agent. Although this reagent was previously thought to be unreactive towards boronic acids, we found that aqueous basic conditions were sufficient to activate HSA in-situ, resulting in the desired conversion of arylboronic acid to primary anilines at ambient temperature (Scheme 1). The reaction shows broad scope for aromatic boronic acids. High yields were obtained for electron-rich, electron-neutral and moderately electron poor substrates. Boronic acids substituted with strongly electron withdrawing substituents (e.g. CF3) required heating in order to achieve acceptable product yields. The reaction conditions are compatible with a variety of functional groups (e.g. halogens, alcohols, alkenes), but substrates bearing carbonyl substituents either decomposed or failed to react under the conditions.
The simplicity of this amination method and our previous observation that THF was an equally suitable solvent as acetonitrile, prompted us to test it in combination with various methods commonly used to prepare boronic acids. Thus, we developed a one-pot protocol for lithium-halogen exchange / boronation / amination (Scheme 2). Product yields were comparable to those starting from isolated boronic acids.
In view of the fact that various methods for the preparation and purification of boronic acids result in their production in protected form, we next tested the compatability of our amination method with various boronic acid derivatives (Scheme 3). We were pleased to observe that various boronic esters and even (with the addition of fluorophilic silica gel) trifluoroborates are equally compatible under the reaction conditions, affording the product in comparable yield to that for reaction of the free boronic acid.
In a final synthetic investigation, we combined our amination method with DoM, in order to provide a convenient source of electrophilic ‘+NH2’ for that reaction (Scheme 4). Although a tertiary amide substrate failed to undergo amination, secondary amides as well as a variety of O-based DMGs were found to be compatible with our reaction conditions, affording the desired products in very good to near-quantitative yields.
In order to obtain mechanistic insight into the process, we performed a DFT computational study on several likely reaction pathways. Our results suggest that an initial complexation of doubly-deprotonated HSA and the boronic acid occurs to form a borate. This species undergoes aryl-1,2 B to N migration with simultaneous loss of the sulfate moiety. This migration appears to be assisted by a two-fold hydrogen-bonding arrangement between one of the sulfate oxygens and the two hydroxyl groups on the boron atom. Comparison with the analogous, higher energy mono-deprotonated pathway suggests that the base performs a critical role in activating the HSA reagent.