Reports: UNI353605-UNI3: Synthesis of Cationic Nickel(II) Complexes Containing Hemilabile Groups for Use as Alkene Hydrogenation Catalysts
Abby R. O'Connor, PhD, The College of New Jersey
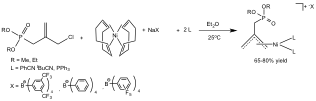

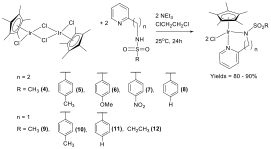
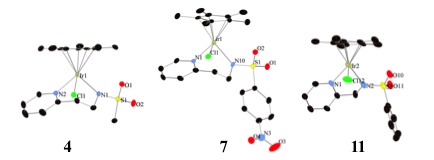
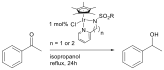
Abby R. O'Connor, PhD, The College of New Jersey




Copyright © American Chemical Society