Reports: DNI652157-DNI6: Non-adiabatic Dynamics of Small Polyphenyls Studied with Time-Resolved Spectroscopies
Arthur E. Bragg, PhD, Johns Hopkins University
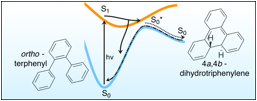
Figure 1: Summary of the photochemical dynamics of o-terphenyl.
Earlier this year we published a comprehensive description on the photochemical dynamics of OTP (J. Phys. Chem. A 118, 3913-3925 (2014)). This work characterized dynamics occurring on timescales ranging from femtoseconds to nanoseconds that can be ascribed to three distinct processes (see Figure 1): (1) ultrafast non-adiabatic excited-state relaxation (including cyclization) on solvent-dependent timescales of 1.5-4 ps (solid branching arrow); (2) vibrational relaxation of the nascent photoproduct, 4a,4b-dihydrothriphenylene (DHT), on solvent-dependent timescales of 10-25 ps (dotted line); and (3) thermally activated ring-reopening of DHT on a timescales of 46 nanoseconds (dot-dashed line). Characterization of process (1) extended beyond our publication in
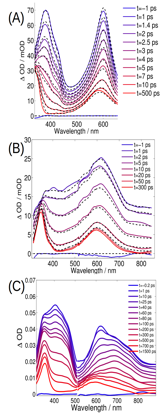
We have also completed an investigation of the photo-induced dynamics of two related molecules, 1,2,3-triphenylbenezene (TPB) and o-quaterphenyl (OQTP), in order to understand the influence of molecular structure on the course of non-adiabatic photocyclization. The dynamics of TPB and OTP are highly similar (Figure 2A and B), but with non-adiabatic relaxation of TPB occurring on a somewhat longer timescale of 7 ps. In contrast, OQTP exhibits more complex dynamics (Figure 2C) on two timescales (~50 and 400 ps), revealing the participation of three different photochemical states. Quantum chemical calculations indicate that the excited-state potential energy landscape of TPB is qualitatively similar to that of OTP, but that OQTP has an excited-state minimum with a geometry (a quinoidal configuration) far removed from that of the cyclization conical intersection; we expect that the presence of this minimum most likely explains the differences in excited-state dynamics relative to OTP and TPB. We are currently writing up these findings for submission. We have also begun to examine how methylation at various sites on OTP alters the non-adiabatic cyclization rate. Although we observe remarkable similarities with OTP for some structures, methylation of other sites significantly modifies the excited-state spectroscopy and dynamics of the chromophore – in some cases shutting off cyclization entirely. We are continuing to examine the nature of substituent effects for these systems.
Figure 2: Time-resolved spectral dynamics of (A) o-terphenyl (OTP), (B) 1,2,3-triphenylbenzene (TPB), and (C) o-quaterphenyl (OQTP).
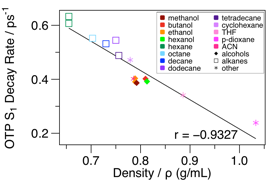
We have also been using o-terphenyl as a model system for investigating how solvent-solute interactions influence the dynamics that occur along low-barrier chemical reaction pathways. Isoviscous, temperature-dependent measurements indicate very low energy barriers (~100 cm-1) along the relaxation pathway of excited OTP. Solvent-dependent measurements of the OTP excited-state decay rate further reveal very weak correlations with solvent polarity and dielectric strength, suggesting that regions of the potential-energy surfaces that control the excited-state decay rate are weakly affected by electrostatic interactions with the solvent. However, significant correlations between reaction rates and solvent density across a wide collection of solvent types (i.e., polar to nonpolar, protic to non-protic, see Figure 3) reveal that solvent influences on excited-state dynamics occur primarily through mechanical interactions that are most likely collisional in nature. We noted this trend in our comprehensive publication on OTP photochemistry earlier this year, but are now generalizing this result by investigating solvent dependence of various OTP analogs in a broader range of solvents. Strong correlations between rate and density and poor correlations with solvent viscosity do not match expectations of theories of low-barrier reaction dynamics. We are currently looking into using molecular dynamics simulations to understand the origins of the significant density dependence, with the hope of providing a comprehensive explanation for this effect for publication in the coming year.
Figure 3: Solvent-dependence of the o-terphenyl photocyclization rate.
Research activities described above have greatly impacted the professional development of the graduate students involved due to the breadth of their experiences in the laboratory (ranging from spectroscopic measurements, theoretical calculations, and synthetic preparations). Furthermore, one student was supported through this award to present a poster of research findings at the Gordon Research Conference on Atomic and Molecular Interactions in July of 2014. Funds from this award were utilized primarily to support students working on projects associated with this grant over the last year.
The PI's career has also greatly benefited from activities associated with this project. He presented results of this project through a young investigator talk at a Gordon Conference this past summer, raising his visibility in the fields of chemical dynamics and photochemistry. Progress in this research over the award period has led to new and broader research directions from the original proposal and the PI has been developing and submitting proposals to other funding programs (e.g. NSF, DOE) to garner support for continuing research in this area. Furthermore, this work has led to collaborative exchange with physical organic chemists both at Hopkins and elsewhere on problems relating to non-adiabatic photoswitches and photochemistry - thus opening doorways for expanding the scope of work in the PI's lab.