Reports: ND651898-ND6: Understanding Interfacial Phenomena Related to Enhanced Oil Recovery
Jeffrey R. Errington, PhD, State University of New York at Buffalo
The goal of this project is to develop a better understanding of wetting phenomena in rock-oil-water systems. We are particularly interested in the manner in which various components within crude oil impact wetting behavior. Many have speculated that the heavy asphaltene fractions of crude oil are responsible for altering the wettability of a rock from preferentially “water-wet” to preferentially “oil-wet”. It has been suggested that during the aging process charged components within the asphaltene fraction, such as acids and bases, adsorb onto the rock surface, thereby changing the surface chemistry of the rock. The specific aims of this project include (1) developing a means to compute the wetting properties of immiscible liquids (e.g. oil and water) near a solid surface (e.g. rock) via molecular simulation and (2) apply these techniques to quantify the extent to which carboxylic acids, which are commonly found within the heavy asphaltene fractions of crude oil, alter the wetting properties of the rock-fluid interface. During the last year of the project we established the bulk phase coexistence properties of a model oil-water system and began a series of wetting calculations.
Bulk Phase Behavior of Model Water-Octane System
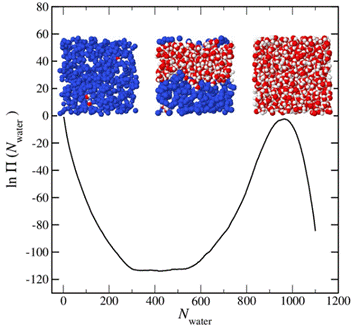
The wetting behavior of a system is intimately connected to its bulk phase behavior. Therefore, we first performed a series of simulations to determine the bulk saturation properties of the model water-octane system we are working with. This system exhibits rather interesting phase behavior, showing liquid-liquid (LL) phase separation at relatively high pressures and two liquid-vapor (LV) phase separation regions (water-rich LV and octane-rich LV) at relatively low pressure. These phase diagrams were constructed using a two-step process. In the first step, we complete so-called direct calculations that provide a single saturation point. For example, to locate a LL saturation point we completed a simulation within the grand canonical ensemble at fixed temperature, water activity, octane activity, and volume. Advanced sampling techniques were used to obtain the water molecule number probability distribution, which was used to determine coexistence conditions. An example distribution from a simulation completed at a temperature of 450 K is provided in the figure below. The snapshots show typical simulation configurations at select values of the water number.
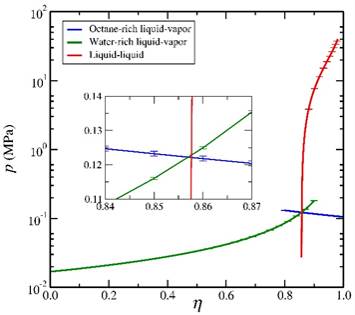
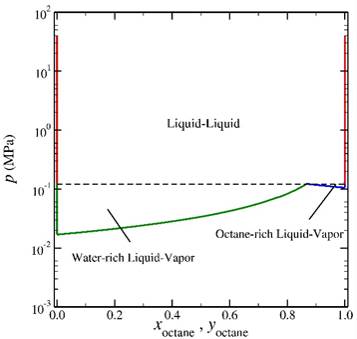
The second step of the process involved the use of various expanded ensemble techniques to trace saturation lines over wide ranges of temperature and composition. The figures below provide representative phase diagrams for the water-octane system at 350 K. The first provides the relationship between the pressure and the activity fraction of octane along the LL and two LV saturation lines. The three curves meet at a triple point (inset). Note that we follow the saturation lines slightly into the metastable regions such that we can accurately locate the triple point. The second figure provides the traditional pressure-composition phase diagram for the water-octane system. The techniques employed enable us to determine the saturation points to a relatively high level of precision. As discussed below, we are now completing interfacial simulations in which we determine wetting properties along these bulk saturation lines.
Finally, we note that we have also completed a series of calculations that show the impact of carboxylic acids on water-octane LL phase separation. In short, we have constructed saturation lines that show how coexistence properties evolve with increasing carboxylic acid activity at fixed temperature and pressure. These data will serve as input for a future set of interfacial simulations.
Water-Octane Wetting Studies
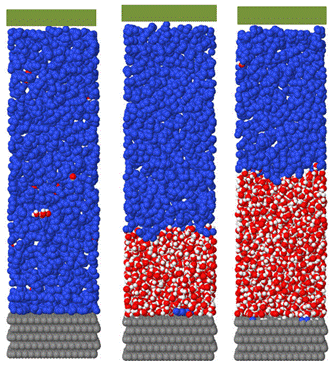
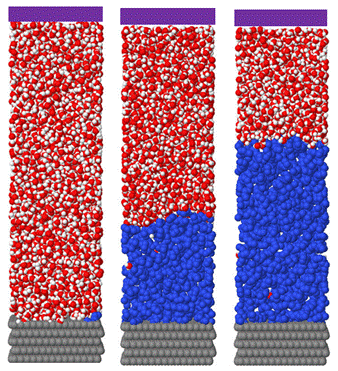
During the first year of the project we developed simulation techniques that enable one to determine the wetting properties of a liquid-liquid phase separating system at a solid surface, and implemented these techniques with a model Lennard-Jones system. We are now employing these methods to capture the wetting behavior of the water-octane system described above at graphite and silica surfaces. These simulations also proceed via a two-step process, with direct calculations followed by various expanded ensemble simulations. In this case, the direct calculations provide an interface potential, which is a well-known construct within the wetting field. Two variants of these calculations are completed: one in which a water film is grown from the surface of interest in a mother octane phase and another in which an octane film is grown from the surface of interest in a mother water phase. Snapshots from simulations focused on the former are provided immediately below, followed by those related to the latter. All snapshots feature a graphite surface placed at the bottom of the simulation cell and a structureless control surface located at the top of the simulation cell. Collectively, these simulations provide the water-octane interfacial tension and the contact angle of a water droplet at the surface of interest in a mother octane phase.
Training
One Ph.D. student, Wenjing Guo, has been fully supported (stipend and tuition) by this project. Wenjing recently began the fourth year of our graduate program. This past academic year she focused almost exclusively on research activities. She has studied the bulk phase behavior and interfacial properties of water-oil systems, developed Monte Carlo simulation codes, and completed all of the simulations discussed above. She also drafted a journal article related to the bulk phase behavior study described above.