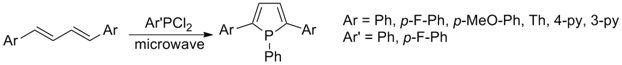Reports: UNI1050237-UNI10: Controlling Morphology and Electronic Properties of Two-Dimensional Organometallic Conjugated Polymers via Orthogonal Polymerization Methods
Katsu Ogawa, Ph.D., California State University (Northridge)
Although the number of reports on new phosphole based Accomplishments in the First Three Years Various synthetic routes were explored for preparation of diarylbutadiene precursors following reported procedures in the literature. McCormack cyclization reaction was utilized for preparation of phosphole oligomers. Instead of utilizing conventional heating methods, the annulation was accomplished by microwave assisted heating of the reaction mixture. Conventional heating tends to give higher temperature at the contact surface and lower core temperature for the reaction mixture. The microwave heating provide the opposite heating profile with higher core temperature with lower temperature at the glassware surface. This inverted heating profile minimized the formation of byproducts at the higher temperature surface while keeping the core temperature above optimal reaction temperature for the product formation. The use microwave significantly improved the product yields and shorten the reaction time to in the order of hours instead of days. Not only did it improved the yields but also resulted in better purity by suppressing both the dimer and polymer formation.
Accomplishments in the Fourth Year and Plans for Future A variety of phosphole oligomers bearing different diaryl groups were synthesized. The major source of byproducts of McCormack cyclization for preparation of phosphole oligomers is sigmatropic rearrangement of aryl groups around the phosphole ring. In attempt to investigate the mechanism of the rearrangement, it was found that heterocyclic aryl groups seem to exhibit better tolerance to rearrangement whereas the phenyl based aryl groups seem to be more susceptible to migration more than hydride shifts. A manuscript was submitted for synthesis and mechanistic investigation of the sigmatropic rearrangement. Currently the manuscript is under revision based on the reviewers comments. In addition to phosphole oligomers, oxides, sulfides, and Pt-complex of above phospholes were successfully prepared. Electrochemical and photophysical properties of these compounds have been investigated and another manuscript for material characterization data is underway.
The oxide, sulfide and Pt-complex of dithienyl phosphole undergo electrochemical polymerization to form a thin layer of conjugated polymeric material on platinum disc electrode. In order to accomplish the goal for the project, dialkoxydiethynylbenzene derivatives bearing a various length of alkyl chains (C6-10) have been prepared for synthesis of Pt-acetylide polymers. After successful synthesis and characterization of the polymers, these materials will be spin coated onto ITO electrode. The change in photophysical and electrochemical properties before and after the electropolymerization of phosphole ligand will be investigated.
The Impact of the ACS PRF UNI Grant on the PI and Students The UNI grant provided essential financial support for PI's research activities. The continuation of the research would have been impossible without UNI grant after the start up funding from the institution was exhausted for purchasing spectroscopic analytical instrumentations. Total of three undergraduate students were actively involved in the project during last year and one of them was fully supported by the grant.