Reports: ND652618-ND6: Nonlinear Light Scattering from Micelles and Emulsion: Development of a Versatile Probe of Structure and Kinetics
Grazia Gonella, PhD, Temple University
Hai-Lung Dai, PhD, Temple University
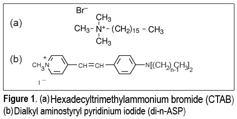
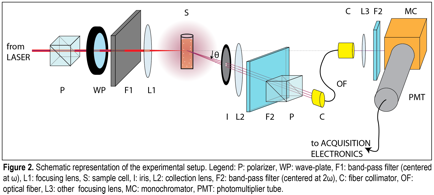
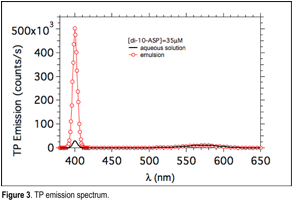
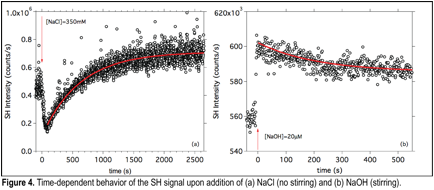
Grazia Gonella, PhD, Temple University
Hai-Lung Dai, PhD, Temple University




Copyright © American Chemical Society