Reports: DNI753294-DNI7: A Fresh Perspective on Admicellar Polymerization: Determining the Effects of Deoxygenation, Monomer Partitioning, and RAFT Polymerization on Thin Film Formation
Adam E. Smith, PhD, University of Mississippi
One of the major challenges leading to a lack of fundamental
knowledge regarding the admicellar polymerization process is the difficulty of
synthesizing enough polymer for detailed kinetic studies and the difficulty of
completely extracting the polymer from the substrate without degradation
occurring; thus the properties (i.e., molecular weight, tacticity, branching) of
polymers synthesized by admicellar polymerization are virtually unknown. A
significant effort in the first year was devoted to investigate proper
techniques for synthesizing sufficient polymer for analysis. This included
investigating both porous silicas of varying surface area and non-porous glass
beads as the substrate. In our studies, we have utilized cetyltrimethylammonium
bromide (CTAB) as the surfactant, precipitated silica (Hi-Sil 233) as the
substrate, styrene as the monomer, and either 2,2'-azoisobutyronitrile (AIBN)
or 4,4-azobis(4-cyanopentanoic acid) (V-501) as a radical initiator. We
utilized a monomer to surfactant ratio of 2:1 and varied the monomer to
initiator (M:I) from 15:1 to 1000:1. After polymerization, the formed polymer
film was isolated from the silica via Soxhlet extraction with THF.
Effect of Oxygen on Admicellar Polymerization In previous investigations, admicellar polymerizations
utilized a low monomer-to-initiator ratio relative to emulsion polymerization.
Our hypothesis is that the requirement for a high initiator concentration stems
from the presence of oxygen (a known radical inhibitor) and can be overcome by
the removal of oxygen from the polymerization solution. In order to investigate
this hypothesis, we deoxygenated the solution by purging the headspace with
nitrogen gas. Immediately prior to removing the nitrogen purge, deoxygenated
styrene was added to the solution and the reaction flask sealed. After
polymerization, the polymer modified silica was dried and analyzed by TGA to
determine the mass of the polymer film. Additionally, the polymer film was
extracted from the silica and analyzed by gel permeation chromatography.
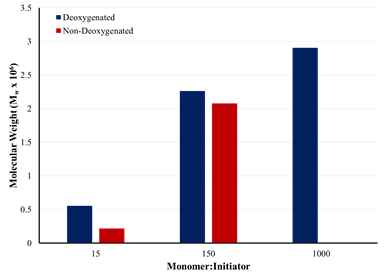
Figure 1 shows the results of preliminary studies on the
effect of deoxygenation on the molecular weight of the polymer formed by
admicellar polymerization using AIBN as the radical initiator. At M:I ratios of
15:1 and 150:1, the molecular weight of the isolated polymer formed in the
deoxygenated system is larger than that formed in the presence of oxygen. At a
M:I ratio of 1000:1, insufficient polymer was extracted from the polymerization
mixture containing oxygen for analysis. These results are thought to be due to
the termination of polymerization by oxygen diffusing into the adsorbed
bilayer. Since AIBN is water-insoluble, the initiator partitions to the
hydrophobic core of the bilayer. Once the polymerization is started, monomer adds
to the active radical until the growing polymer chain is terminated by either
another radical or a radical inhibitor such as oxygen. In the non-deoxygenated
polymerization solution, oxygen may diffuse into the adsorbed bilayer,
prematurely terminating polymerization and leading to suppressed molecular
weight as shown in Figure 1.
Figure 1. Effect of deoxygenation on the molecular weight of
polymer films formed by admicellar polymerization.
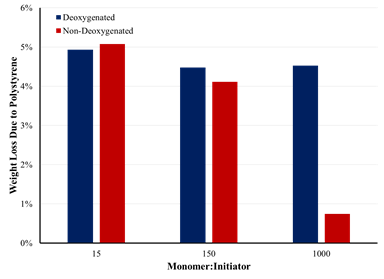
The effect of deoxygenation may also be seen by thermogravimetric
analysis (TGA) on the modified silica. Figure 2 shows the weight loss
associated with the polystyrene film formed on the surface on the silica. Based
on system loading, the weight loss expected if all styrene monomer is
incorporated into the polymer film is roughly 5%. At an M:I of 15:1, both
systems show that essentially all the styrene is incorporated into the polymer
film. At 150:1 and 1000:1, the deoxygenated system generates a greater mass of
polymer film than the polymerization performed in the presence of oxygen. The
TGA reaffirms the observation of very little polymer formed in the presence of
oxygen at an M:I ratio of 1000:1.
Figure 2. Effect of deoxygentation on polymer film mass at
varying monomer to initiator ratios.
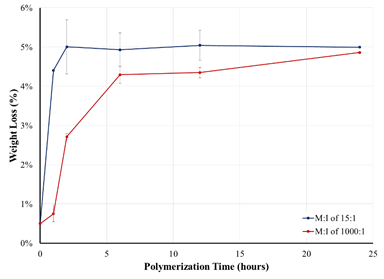
Kinetics of Admicellar Polymerization Another focus for the first year of the project has been to
investigate the kinetics of admicellar polymerization. Utilizing the same
system described above, we utilized TGA to determine the weight loss attributed
to polystyrene for M:I ratios of 15:1 and 1000:1 as a function of
polymerization time. As Figure 3 shows, polymerizations performed at a 15:1
ratio reach completion after two hours while polymerization utilizing a M:I
ratio of 1000, require between 8 and 24 hours to reach completion.
Figure 3. Kinetics of the admicellar polymerization of
styrene for M:I ratios of 15:1 and 1000:1.