Reports: DNI753287-DNI7: Combinatorial Approaches for the Discovery of Stimulus-Responsive Supramolecular Gels
Jonathan Pokorski, PhD, Case Western Reserve University
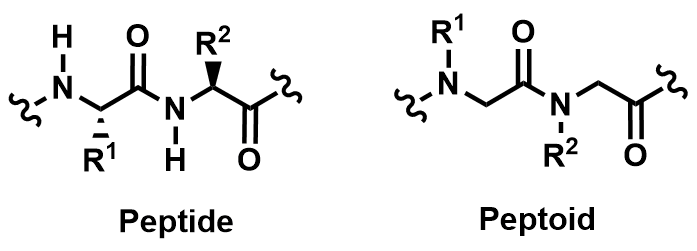
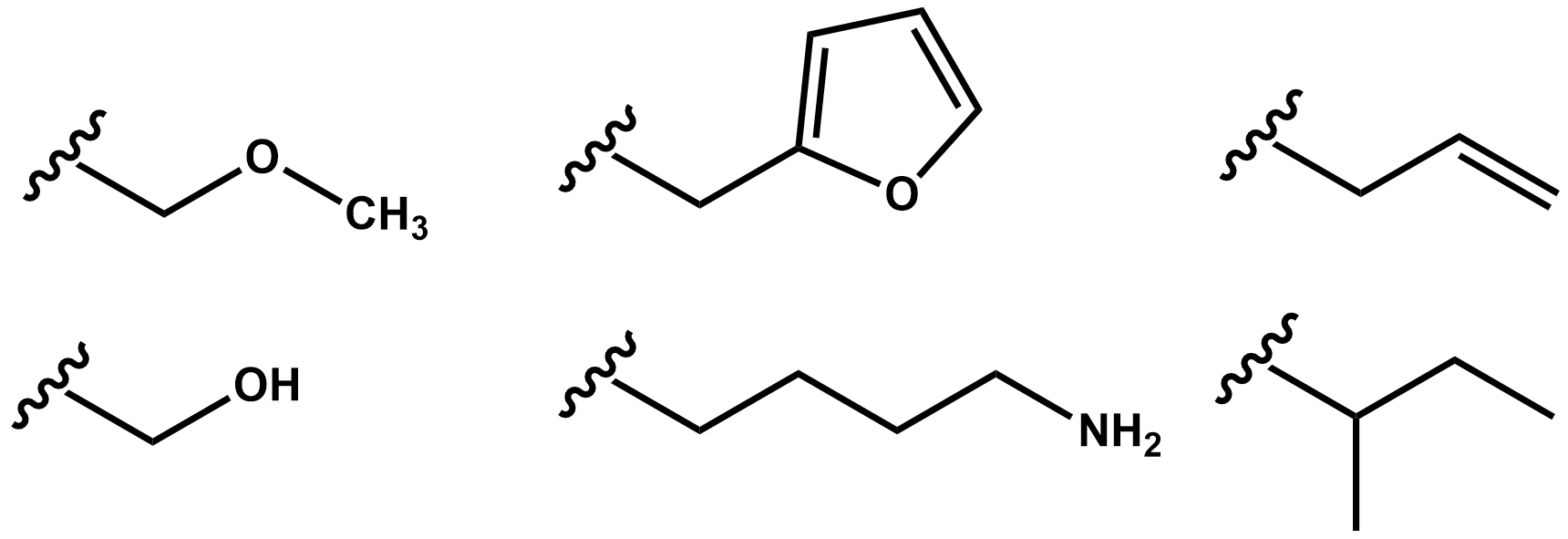

Jonathan Pokorski, PhD, Case Western Reserve University



Copyright © American Chemical Society