Reports: DNI553648-DNI5: Gasoline Selective Fischer-Tropsch Synthesis in Structured Catalytic Reactors
Georgios Bollas, PhD, University of Connecticut
A novel method based on redox exsolution for the synthesis of nanosized catalysts, and the synthesis of multilayered structured catalysts is explored in this project. FTS selectivity enhancement is explored at three levels: (a) experimentation using monolith reactors; (b) highly dispersed FTS catalysts formulated using exsolved Co nanoparticles of controlled size; and (c) bifunctional catalysts formulated via layering of the supported Co nanoparticles and microporous zeolite, to control diffusion phenomena and selectivity in FTS.
This ACS-PRF DNI project supports one graduate student and has served as an undergraduate research study for two undergraduate students of the Chemical and Biomolecular Department at UConn. The main research effort started in January (instead of August) due to immigration paperwork delays in the graduate student’s Visa application. Nonetheless, the project is on track, with the main effort focused on the control of the crystallite size of the Co catalyst at high Co loadings.
Task 1: Experimental Setup
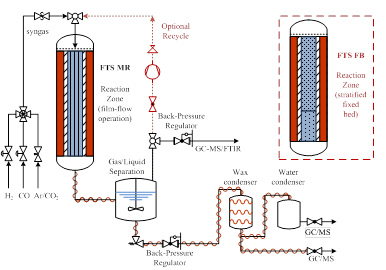
For the first task of the first year of the project, the work focused on setting up a micro-reactor capable of operating as a structured (monolith-based) reactor or a standard fixed bed. The developed experimental facility consists of a tubular reactor for the monolithic catalyst; gas-liquid separator; heating and cooling zones for the product streams; and wax and water condensers for the collection of the heavy and light liquids and the gas product. The process setup is shown in Figure 1 The experimental facility is capable of operating in microreacror or stratified fixed bed mode (MR and FB, respectively – Figure 1) to explore the advantages offered by the microreactor better mass and heat transfer and the selectivity improvements of the bi-layered catalysts. Synthesis gas (H2, CO mixture at a ratio of 2) is fed with He to control space velocity and syngas partial pressure. On-line steady-state gas analysis is performed using a 4900 micro GC (Varian) equipped with 10 m PPA column and 10 m molecular sieve column. The liquid product is analyzed with GC 7890A (Agilent) with a FID detector and an HP-5 column. The wax product is first dissolved in CH2Cl2, then analyzed in the GC 7890A. The reactor is operational at both FTS MB and FB modes.
Figure 1: Experimental microreactor setup for FTS testing.
Task 2: Conventional and Structured Catalysts Preparation
The method of redox exsolution was explored on Al2O3 supported Co catalysts prepared using wet and dry impregnation methods. The catalysts are synthesized using Co(NO3)2·6H2O (99%, Sigma Aldrich) dissolved in DI water, then mixed with γ-Al2O3 or α-Al2O3 (precalcined up to 1550°C). The mixture is dried at 120°C for 12 h followed by calcination at 400-1200°C for 4-12 h. After calcination, the catalyst is reduced in a tube furnace at 600-800°C for 5-12 h with 500 sccpm N2 flow and 30 sccpm H2 flow Monolith catalysts were prepared following similar methods. The monolith support used was cordierite with a cell density of 400 cpsi (1 mm ID channels). A catalyst slurry is prepared by dispersing 26.45 g Al2O3 (γ phase, 99.7%, 3 micro APS powder, Alfa Aesar) and 32.83 g colloidal alumina binder (pseudo-boehmite, AlOOH, 20 wt%, 50 nm, Alfa Aesar) in a 32.65 g Co(NO3)2·6H2O (99%, Sigma Aldrich) with 140 ml DI water. The slurry is coated on the monolith channel surface (coat thickness can be controlled by repeating the dipping process), dried at room temperature while rotating for 2 h and dried at 120°C for another 2 h. Then the monolith catalyst is calcined at 400-900°C for 4 h and reduced at 600-800°C for 4 h with 500 sccpm N2 flow and 30 sccpm H2 flow. Following these synthesis methods, an array of catalysts with considerably different properties was generated. The catalysts were first characterized using TGA, XRD, XPS, SEM and TEM. With temperature programmed reduction in a Pyris 1 (Perkin Elmer) thermogravimetric analyzer, the loading of the Co was estimated, which was further validated (in terms of surface properties) with XPS (VG Scientific). XRD (Bruker D8 Advance power XRD, equipped with LynxEye-super speed detector) was used for phase analysis and crystallite size of the impregnated Co. As anticipated, significant sintering effects from the high-temperature treatments were observed and an overall methodology for controlling the Co crystallite is in progress. To isolate the effect of sintering and agglomeration, very low loadings of CoO on Al2O3 were studied. In these samples, it was shown that exsolution is feasible and the crystal size and dispersion of the active phase can be controlled via high temperature treatment in a redox environment. Figure 2 presents the SEM of a 3wt% CoO impregnated on α-Al2O3 (precalcined at 1550°C) calcined at 1200°C and reduced at 800°C. For comparison the same catalyst calcined at 800°C and reduced at 600°C is also shown (Figure 1(c)). The high-dispersion material is currently being tested in the reactor developed in Task 1 and a high loading and higher surface area material is in preparation.
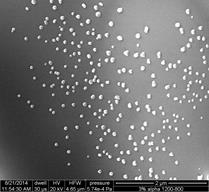 | 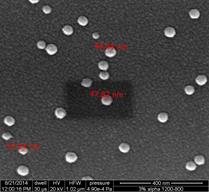 |  |
calcined at 1200°C and reduced at 800°C | calcined at 800°C, reduced at 600°C | |
Figure 2: CoO/α-Al2O3 (precalcined at 1550°C): (a) and (b) show the dispersion of the Co phase after high temperature treatment; (c) shows the same catalyst after cacination at 800°C.