Reports: UNI753041-UNI7: Synthesis of Ester and Amide Functionalized Polyethylene: New Polyolefin Architectures, Their Resulting Thermal and Mechanical Properties, and Controllable Depolymerization
Nicholas J. Robertson, PhD, Northland College
Our research proposal focused on developing a synthetic route to new functionalized polyethylenes with ester or amide linkages at regular, but increasing methylene spacing. We proposed achieving this by synthesizing dialcohols (diols) or diamines with increasing methylene lengths, and then polymerizing viadehydrogenation using the Milstein catalyst. Shortly after receiving our funding two separate manuscripts were published concurrently that outlined the synthesis of these materials. One used an acyclic diene and the other used a ring-opening metathesis polymerization pathway to prepare the polyesters with long methylene spacers between ester linkages. This did not mimic our approach exactly, but it did enable them to answer the core focus of our proposed study to elucidate the impact of methylene spacing between ester units on thermal and mechanical properties. We therefore needed to change the direction of our project midstream.
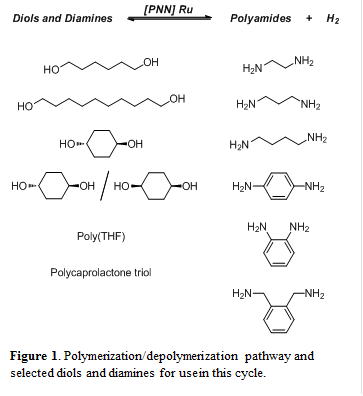
Given our previous successes with polymerizing diols to polyesters, we became interested in polymerizing a variety of diols and diamines to form polyamides that could exhibit useful mechanical properties. We began synthesizing these polyesters and polyamides by polymerizing diols as well as diols and diamines using the Milstein catalyst, which is a ruthenium complex supported by a PNN pincer ligand ([PNN] Ru). Figure 1 shows a variety of diols and diamines that we are currently polymerizing in varying combinations to form high molecular weight polyamides. Excitingly, all combinations of these exhibit activity in the form of vigorous hydrogen evolution and increasing viscosity, which suggests that polymerization viadehydrogenation is occurring for all of these reactions. However, many of the polyamides are proving to be insoluble in a variety of solvents, which is impeding the detailed characterization of these materials. We are exploring numerous solvents commonly used for plastics processing in order to better characterize these polymers, but also so that we can cast films for mechanical testing. One of our goals with this work is to establish a relationship between the chemical structure of the monomers and the mechanical properties of the resulting materials.
We recently published a manuscript demonstrating that [PNN] Ru pincer complexes were reversible and could hydrogenate polyesters and polycarbonates to diols as well as glycols and methanol, respectively. So in addition to synthesizing the polyesters and polyamides briefly outlined here, we are also investigating the ability to chemically recycle the resulting materials through hydrogenation. Our previous work showed that these [PNN] Ru complexes should be able to depolymerize our new materials through exposure to high pressure hydrogen gas (reverse reaction shown in Figure 1). However, the insolubility of some of these polyamides is precluding their depolymerization. So we are focusing on exploring more forcing conditions (higher temperatures and pressures) and alternative solvents for this depolymerization pathway, and we have had some recent successes to support the feasibility of this pathway.
In addition to linear polyesters and polyamides we are investigating crosslinking reactions through the combinations of diols and trialcohols with triamines in the presence of [PNN] Ru. Some of these combinations may exhibit similar properties to polyurethanes, while offering a more environmentally benign pathway to robust materials given the hazards associated with isocyanates. Moreover, with the reversibility of [PNN] Ru catalysts, we are confident that if we can find the right solvents to swell these materials and conditions for hydrogenation, we can develop a new route to crosslinked materials that are chemically recyclable via hydrogenation. Many chemically recyclable crosslinked materials rely upon dynamic covalent bonds (e.g. Diels Alder reactions) to enable the reversal of crosslinks. However, these systems are often limited by the necessity of specific functional groups and monomer structures. Our pathway would enable a large variety of different monomer structures to better allow for optimization of the resulting material properties while still being able to chemically recycle the polymers.
Lastly, there are a variety of commercially available polyols commonly used in the synthesis of polyurethanes. Poly(tetrahydrofuran) and polycaprolactone triol are two such examples, and each system is available in a variety of starting molecular weights. We are beginning to investigate the ability of [PNN] Ru complexes to tie these into polyester and polyamide systems viadehydrogenation, and then chemically recycling them back to polyols through hydrogenation. These polyols are also exhibiting activity through rapid hydrogen evolution and increasing viscosity during the polymerization stage. However, we have again encountered solubility issues with these systems and are working to circumvent this challenge.
In summary, we have made significant progress on the development of a platform system through which a variety of linear and crosslinked materials can be synthesized by dehydrogenation and subsequently depolymerized viahydrogenation. The modular nature of this system should enable specific tuning through the monomer structure, ratios and combinations to best optimize the thermal and mechanical properties of the resulting materials.
Broader Impacts of ACS PRF Funding:
The ACS PRF Undergraduate New Investigator funding has been instrumental in the development of my personal career, but more importantly, it has enabled me to hire four students to date. These students have had life-altering experiences in the lab through their research projects. All four of them have found the hands-on work and the process of research to be so enjoyable that they are now planning on pursuing careers in the chemical industry or graduate school. In my lab I routinely discuss what we are doing with my students and we make research plans together, but it is they who are doing all of the lab work. I have seen these lab experiences increase my students’ critical thinking abilities, their skills in working with their hands, and their abilities to multitask. But above these benefits, I have seen all of them experience increased self-confidence as they are believing more in their abilities. These are exceptionally valuable outcomes for these students.











