Reports: ND552305-ND5: Visualization of Hydrocarbon-Surfactant Interactions at the Molecular Level
Jayne C. Garno, Louisiana State University
Protocols with Immersion of Nanoring Test Structures Prepared with Organosilanes
The 3D nature of the test structures provide an opportunity for visualizing the interactions of headgroups and side chains of the molecules with surfactants. Surfactants will attach onto the top or sides of the patterns, or intercalate between hydrocarbon chains with different orientations depending on the charge nature of the molecule. The selection of solvents and concentration parameters of surfactant solutions are also experimental variables to be tested since experiments are accomplished in liquid media.
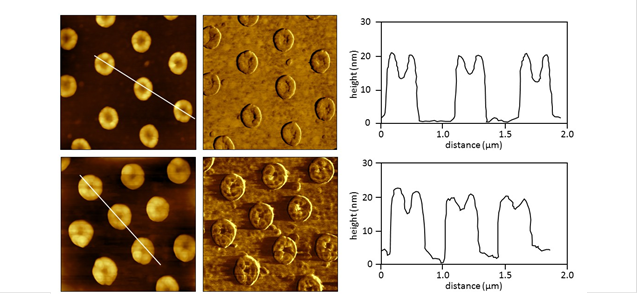
Figure 1. Test platform of oligoethylene-glycol functionalized nanorings immersed in (top row) water and (bottom row) CTAB solution (2 mM). The left column shows AFM contact-mode AFM views of the sample (1.5 × 1.5 µm2), the center panels are the simultaneously-acquired lateral force images acquired in liquid. Cursor profiles correspond to the white lines drawn in the topography frames.
Protocols with AFM topography frames revealed only slight changes in the dimensions of the 3D nanoring test structures after immersion in surfactant solutions, such as cetyl trimethylammonium bromide (CTAB), a cationic surfactant. However, lateral force frames provide compelling views of the location and sizes of adsorbates. An experimental strategy of the research plan was to use nanoscale lithography to prepare model test platforms of hydrocarbons with well-defined composition, orientation and dimensions. Self-assembled monolayers (SAMs) of n-alkanethiols and n-alkylsiloxanes provide excellent model systems for studies of surface reactions, with well-known dimensions and interfacial chemistry.
Immersion of Nanografted Organothiol Test Structures into Surfactant Media
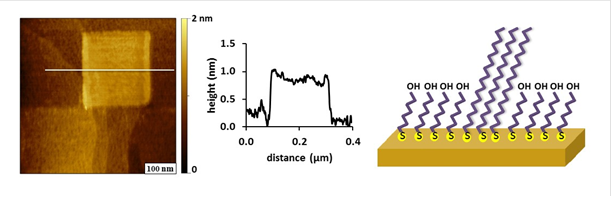
Nanografted patterns of n-alkanethiols were also used to generate surface test structures (Figure 2). Molecules that were selected to prepare nanografted patterns were longer than the matrix monolayer, to present patterns with edges and corners for visualizing surface changes after immersion in surfactant solutions. Successive changes in surface topography can be viewed after each step: inscribing SAM patterns, rinsing, and introducing surfactants. With in situ writing, the test patterns of hydrocarbon chains remain in a carefully controlled environment and solutions within the liquid cell can be exchanged by rinsing to introduce fresh reagents.
Figure 2. Test structure of a nanografted square of octadecanethiol within a matrix monolayer of 11-mercaptoundecanol. The mage was acquired with contact mode AFM in liquid media.
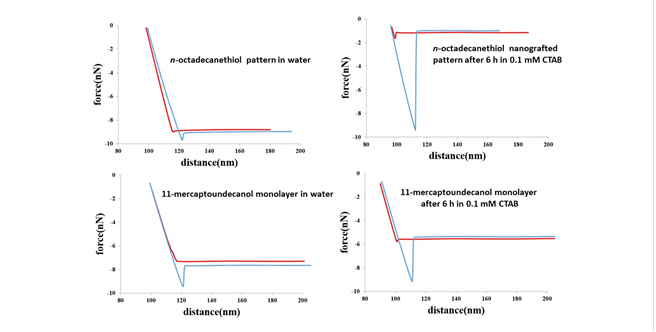
Surfactants were observed to bind throughout areas of both methyl- and hydroxyl- terminated films, exhibiting little change in overall surface topography. However, experiments with force-distance spectroscopy demonstrated that surface changes occurred and provided information of the effects of surfactants for changing interfacial chemistry (Figure 3). Minimal capillary and adhesive interactions were detected for methyl and hydroxyl terminated films immersed in aqueous media. However, as time progressed, the adhesive interactions between the AFM probe and surfactant-treated surfaces were observed to increase. Further directions for in situ protocols will be to evaluate the use of coated AFM probes with well-defined chemical functionality.
This research targeted a new direction for in situ protocols for visualizing surface reactions using time-lapse AFM. Snapshots of surface changes as adsorption took place provide insight for the association of surfactants with surface-bound hydrocarbons as influenced by the effects of pH, ionic strength and selected solvent media.
Figure 3. Force-distance curves for n-alkanethiol surfaces before and after immersion in CTAB, a cationic surfactant.
Educational Impact - Mentoring and Career Development
Training opportunities for both graduate and undergraduate students were provided by this award. Students were trained with cutting-edge experimental and analytical skills as they learned to design and develop new experimental protocols with liquid AFM. During the time span of the New Directions award, seven graduate students received stipends for research positions. Among these, six were female graduate students (including two underrepresented minority students). Three of the students completed their PhD studies and accepted academic positions at undergraduate universities. Funds from this grant have been quite valuable for advancing the PI's research program at LSU, providing crucial funds for continuing research activities. The funds appropriated for travel were used to sponsor student poster presentations at nearby regional and national conferences of the American Chemical Society. Fellowship support for graduate students from the ACS PRF New Directions program has increased the laboratory capacity for sponsoring undergraduate chemistry majors for research opportunities. Currently, the PI's group includes six undergraduate researchers who contribute 3-6 hours each week to obtain research credit. Five of the undergraduates are from underrepresented groups, and the sixth is a Caucasian female. Undergraduate participation in chemistry research is critical to retention of students for the future pipeline of career chemists.