Reports: DNI752722-DNI7: Ruthenium and Osmium Alkylidyne Complexes as Catalysts/Initiators for the Ring-Opening Alkyne Metathesis Polymerization
Felix R. Fischer, PhD, University of California Berkeley
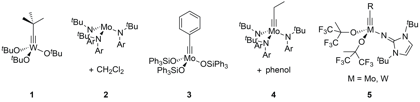
Figure 1. Alkyne metathesis catalysts based on tungsten and molybdenum alkylidyne complexes.
Initial attempts using tungsten and molybdenum alkylidyne complexes 1 to 5 (Figure 1) led to the formation of polymers with broad weight distributions. These highly active catalysts fail to discriminate between the metathesis of ring-strained monomer precursors and unstrained alkynes in the growing polymer chain. Intra- and intermolecular chain-transfer processes ultimately dominate the linear chain-growth process and lead to polydispersity indices (PDIs) in the range of 2–6.
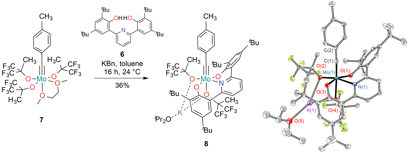
In an effort to increase the selectivity of ROAMP for the activation of strained over unstrained alkynes, we incorporated a permanent electron donating ONO pincer ligand 6 that stabilizes the high oxidation state of the molybdenum carbyne complex and irreversibly blocks one of the catalyst's active sites. Deprotonation of the ONO pincer ligand 6 with potassium benzyl followed by addition to [TolC≡Mo(OR)3(dme)] in toluene quantitatively converted 7 to the desired product 8, by 1H and 19F NMR. The product was isolated in 36% yield after recrystallization from diisopropyl ether at –35 °C. Crystals of 8are stable in air for several hours and can be stored for indefinite time under an atmosphere of nitrogen.
Figure 2. ORTEP representation of the X-ray crystal structure of 8. Thermal ellipsoids are at the 50% probability level. Color coding: C (gray), O (red), F (green), Mo (turquoise). Hydrogen atoms are omitted for clarity.
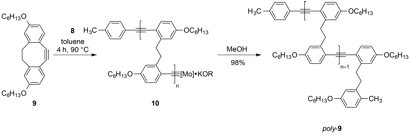
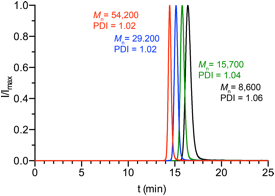
We studied the ROAMP of 3,8-dihexyloxy-5,6-dihydro-11,12-didehydrodibenzo[a,e][8]annulene (9) (Figure 3), a highly solubilized ring-strained alkyne, with 8. Addition of 8 to a solution of 9 in toluene ([9]/[8] = 10) at 24 °C does not lead to the formation of polymeric species within 24 h. 1H and 19F NMR indicate that the ROAMP catalyst 8 quantitatively reacts with a half life of t1/2 < 5 min with one equivalent of 9 to form the initiated complex 10 (n = 1)(Figure 3). Instead, at 90 °C, the initiation reaction is instantaneous and the living ROAMP of monomer 9 in toluene is completed in less than 2 h, as determined by 1H NMR. Precipitation of the resulting polymers in MeOH affords poly-9 in greater than 90% isolated yield. GPC analysis for various monomer/catalyst loadings at 90 °C in toluene shows a polydispersity index (PDI) of ~1.02, the lowest value reported for ROAMP (Table 1). The molecular weights of poly-9 determined by GPC, calibrated to polystyrene standards, are proportional to the initial [9]/[8] loading and show a unimodal distribution (Figure 4). No evidence for branching or the formation of cyclic polymers could be observed by 1H NMR analysis. End-group analysis reveals that GPC overestimates the Mn of poly-9. An estimated correction factor ~0.5–0.7 correlates well with the degree of polymerization determined by NMR analysis and the expected molecular weight based on the [9]/[8] loading.
Figure 3. ROAMP of 9 with catalyst 8.
Table 1. Molecular weight analysis of poly-9.
[9]/[8] |
T (°C) |
Mn theory |
Mn GPC |
Mw GPC |
Xn
|
PDI GPC |
10 |
90 |
4,000 | 8,600 | 9,200 | 11 |
1.06 |
20 |
90 |
8,100 | 15,700 | 16,200 | 23 |
1.04 |
50 |
90 |
20,200 |
29,200 |
30,000 |
47 |
1.02 |
100 |
90 |
40,400 |
54,200 |
55,300 |
99 |
1.02 |
Figure 4. GPC traces of poly-9.
Extensive mechanistic studies of the ROAMP with 8 reveal that the catalyst system meets the stringent criteria for a controlled living polymerization. The initiation of the catalyst is fast and quantitative (ki/kp = 103), the concentration of propagating species remains constant throughout the reaction, all propagating chains grow at the same rate kp, and irreversible termination and chain transfer processes are absent. Furthermore the release of ring-strain stored in the cyclic monomer 9 makes the initiation and the propagation irreversible. Ongoing research is dedicated toward exploring the substrate scope of catalyst 8 and the synthesis of block-copolymer heterostructures through living ROAMP.
In summary, progress over the last funding period involved the synthesis of a molecularly well-defined ROAMP catalyst based on a molybdenum benzylidyne ONO pincer complex [TolC≡Mo(ONO)(OR)]·KOR (R = CCH3(CF3)2) 8. The incorporation of a permanent electron donating tridentate ligand irreversibly blocks one of the catalyst's active sites, prevents undesired alkyne polymerization reactions, and significantly increases its stability towards air and moisture. The catalyst is capable of selectively ring-opening strained alkynes in a controlled polymerization to yield high molecular weight polymers with exceptionally low PDIs (1.02). Mechanistic studies reveal that the ROAMP catalyst 8 meets all the criteria for a controlled living polymerization: the initiation reaction is quantitative and ~103 timers faster than the propagation (ki > kp), the concentration of catalytically active complex is constant throughout the reaction, and all propagating chains grow at the same rate. The reversible coordination of KOR to the propagating catalyst prevents undesired chain termination. The catalyst developed herein provides an unprecedented control and access to highly functionalized derivatives of poly-(arylene ethynylene) for applications in advanced thin-film electronics/photonics, molecular sensing, and nano-patterning.