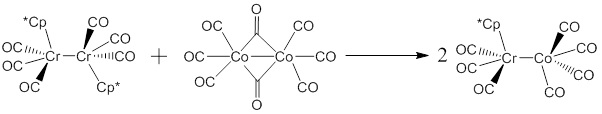
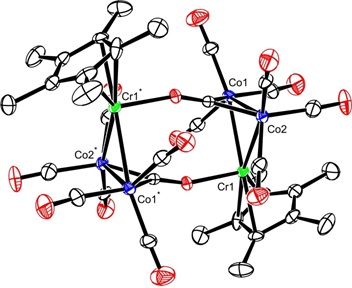
Reports: ND351756-ND3: Catalytic Carbonylation of Ketenes under Oscillating Pressures of Carbon Monoxide
Carl D. Hoff, PhD, University of Miami
The mixed metal dimer appears to react more rapidlywith trimethylsilyl
diazomethane yielding the corresponding ketene but it is prone to decarbonylation