Reports: ND752339-ND7: Mechanical Control of Molecular Encapsulation
Byron W. Purse, PhD, San Diego State University
The goal of this project is to test our hypothesis that polymer-appended molecular capsules will release and take up small molecules in response to mechanical forces, providing a new and orthogonal control for molecular encapsulation with prospective applications in chemical compartmentalization and materials science. A prerequisite for this supramolecular mechanochemistry is that the employed molecular capsules have unusually high kinetic stability. Guest exchange must be induced mechanically from capsules filled with small molecules that are different from those preferred at equilibrium. Success also requires synthetic methods to prepare homogeneous batches of polymer-appended capsules with low polydispersity. Our research plan is to develop syntheses for polymer-appended capsules of sufficient kinetic stability, methods to load them with guest molecules in a kinetically trapped state, purify them, and then use ultrasonication to correlate the forces required for mechanically induced guest release to chemical structure.
During the first year of the project, we investigated synthetic methods to prepare functionalized, self-assembling molecular capsules for polymer attachment, plus we investigated methods to load the capsules with guests under conditions that would give rise to metastable, kinetically trapped complexes. That year of funding supported two Ph.D. students. While potentially suitable polymer attachments were successfully developed in Year 1, problems of reactivity in polymer attachment, scalability of the synthesis, and purification have necessitated changes to the design of the molecules and the synthetic routes. We have made significant progress on addressing these problems during the second year, as will be described below. Furthermore, our results in the first year were very successful on developing methods to separate kinetically trapped molecular capsules from smaller, unencapsulated guest molecules using chromatography. This capability is a prerequisite for many applications of kinetically trapped molecular capsules, including the goals of this PRF-funded project. The purified, kinetically trapped molecular capsules could be induced to release their occupants on cue, in response to an operate-provided stimulus such as a temperature change. These results have now been published.
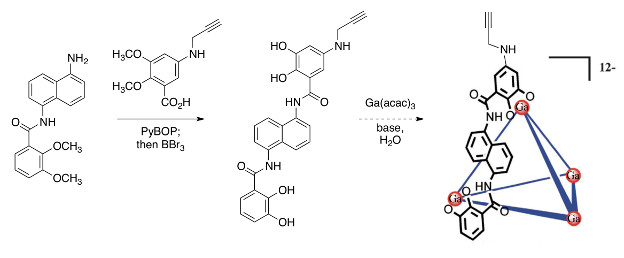
In the first year report, I described the preparation of an amino-functionalized ligand for a coordination capsule designed to be mechanically responsive. Subsequent work has revealed that the amino group reacted sluggishly with carboxy-terminated polymers, and so we sought a better design that would minimize the need for delicate purification after polymer attachment. The CuAAC “click” reaction is a natural choice, and so we developed a modified synthesis that adds a propargyl group to the amine (Scheme 1). We are currently working on a scaled up synthesis and expect to be able to attach polymers and complete the proposed studies within this, the extension year of the project.
For the second approach using hydrogen bonded capsules, we
have examined many possible routes for polymer attachment, and this problem has
remained challenging. For all synthetic approaches, we begin with a
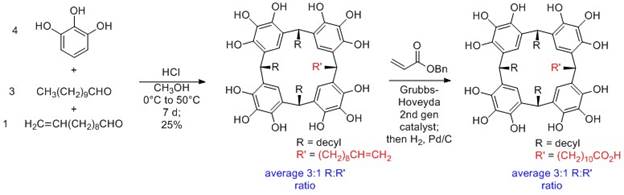
condensation reaction of pyrogallol and either a
mixture of aldehydes or a single type of aldehyde. In the former case (see
Scheme 2), we obtain a statistical mixture of products that must be separated,
a very challenging endeavor with these amphiphilic
molecules. Using this approach, we prepared pyrogallolarenes
with a 3:1 statistical ratio of alcohol-functionalized and purely aliphatic R
groups (not shown), but could not separate them by chromatography. We also
failed to differentiate the aliphatic alcohol by reactivity. The pyrogallol groups are too sensitive to oxidize the
aliphatic alcohol, and differentiation using a reagent such as DPPA failed due
to an exothermic degradative process. Instead, we
synthesized alkene-functionalized pyrogallolarenes.
Initial efforts to differentiate the alkene using radical thiol
addition or epoxidation failed, but we found that
cross metathesis followed by hydrogenation could be used to yield a carboxy-functionalized pyrogallolarene.
Purification of the statistical mixture of products remains problematic, but we
anticipate improvements when polymers are present.
The graduate student responsible for the publication and most
of the pyrogallolarene work was supported on this
grant throughout the completion of her Ph.D. work. She defended successfully in
May 2014. Another graduate student supported on the project has made major
contributions to the coordination capsule work. The project is being continued
by undergraduate researchers and a new graduate student researcher in the Purse
Group at SDSU.
In summary, during the two years of the project funding, we
have completed many key steps needed to create the polymer-appended capsules
and revised our synthetic approaches to overcome significant roadblocks. We have
demonstrated the required stability and purification of the hydrogen-bonded
assemblies. The funding has supported the training of three Ph.D. students, one
masters student, and two undergraduates, lead to one published paper, and been
discussed by the PI at invited lectures. In the next year, we expect to
complete the project and publish the results of the mechanochemistry
studies. As such, this grant is advancing the training of students and the
career of the PI.