Reports: DNI1052146-DNI10: Synthesis of Metastable Solid State Materials Using Organometallic Reagents
Tyrel M. McQueen, PhD, Johns Hopkins University
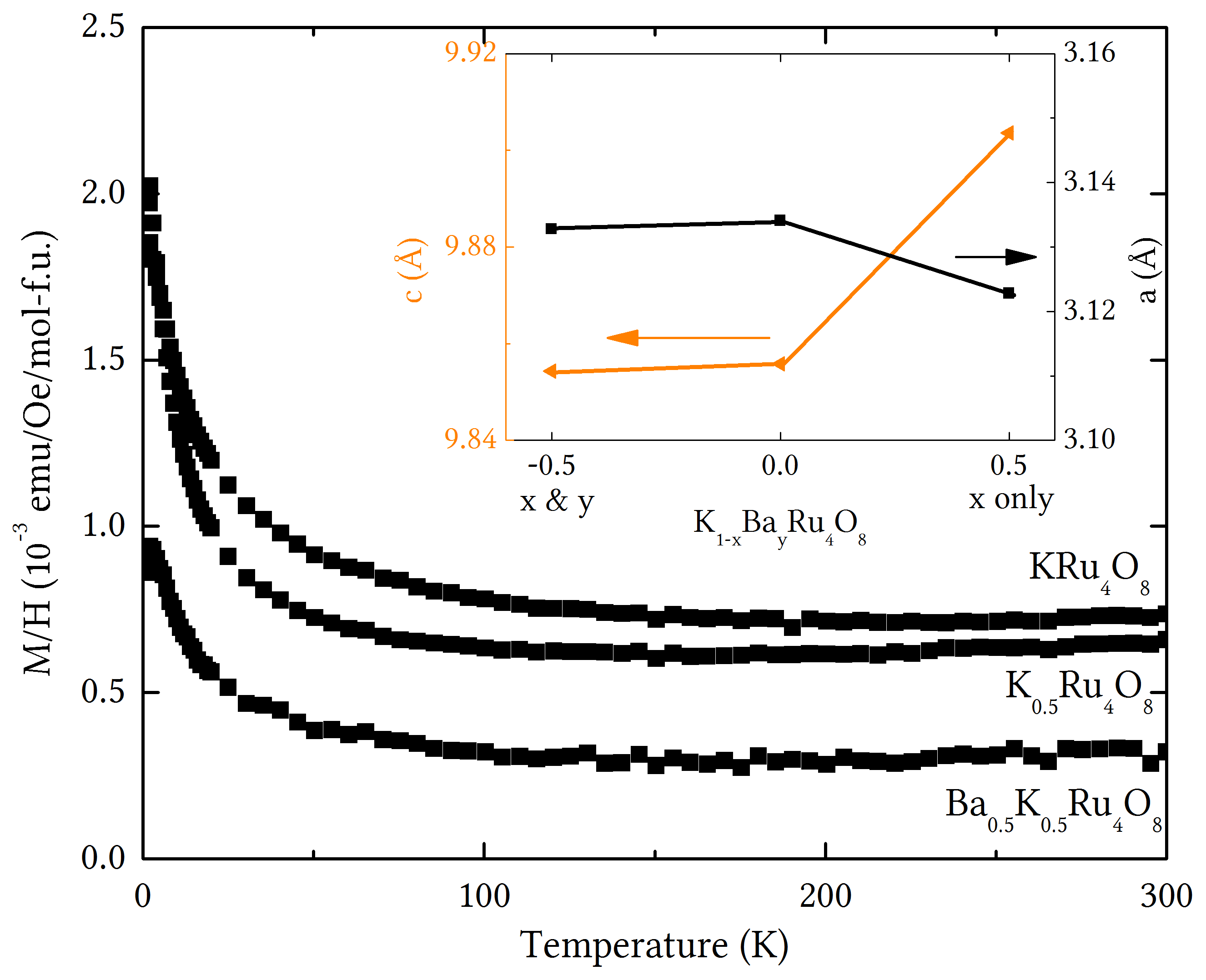
Figure 1: Magnetic properties of the 4d transitional metal Hollandite, K1-xBayRu4O8 at formal Ru oxidation states of 3.88, 3.75, and 3.63, showing only minor variations despite the significant change in electron count. The latter was only possible due to organometallic insertion of barium. Inset: lattice parameters as a function of K and Ba content.
In work with Hollandite-type
materials, we followed our previous report of K1-xIr4O8
to prepare the series K1-xRu4O8. As with the
iridate, all samples were metallic with a weak magnetic response. After the
two-step synthesis of K0.5Ru4O8, which is non-trivial
due to the volatility of potassium and iridium, we were able to systematically insert
barium using a barium-benzophenone radical anion adduct prepared in-situ in THF,
as demonstrated by the systematic change in lattice parameters (Figure 1 inset).
On room temperature removal of potassium from KRu4O8 to
form K0.5Ru4O8, the a lattice parameter
decreases and c increases; however, once barium is inserted to form K0.5Ba0.5Ru4O8,
the lattice parameters return to almost the same values as found for KRu4O8.
Initially this was surprising due to the differing electron counts, but can be
explained as arising from the fact that potassium and barium have virtually
identical ionic radii. While the physical properties of K1‑xBayRu
In our explorations of the reaction
mechanisms of low temperature soft chemistry, we carried out spectrophotometric
monitoring of the kinetics of deintercalation of sodium from Na2IrO3
using iodine in acetonitrile (the system was chosen because of convenient
timescales for the measurements). Representative data showing the reaction
progress (i.e. x in Na2-xIrO3) as a function of time for
two different initial values of the ratio [I2]0/[Na2IrO3]0
are shown in Figure 2. There are two surprising features in the data. First is
a pronounced 'kink' in the reaction progress, which occurs at the same value of
x independent of the rate of solution stirring. Such a kink must arise due to a
change in the rate limiting step of the reaction. Second, the initial rate of
the reaction increases dramatically upon mixing, indicating that the pre-'kink'
rate limiting step at least partly involves diffusion in the liquid solution,
instead of being solely controlled by the rate of ion diffusion in the solid.
A more detailed view of the 'kink' is
obtained by plotting the value of x at which the 'kink' occurs as a function of
the ratio [I2]0/[Na2IrO3]0,
Figure 3. There is a near-perfect linear relationship between the two
parameters, with a slope and intercept indicating that the 'kink' occurs when
the reaction reaches ~60% of what it would be at completion (each I2
can remove two Na ions). We are presently completing experiments to understand
the origin of this unusual behavior: does it arise due to triiodide formation?
Or does it have to do with a switch to the rate limiting step in the solid
state? Preliminary experiments using Br2 in place of I2
suggest triiodide is not important. But if it is instead the latter explanation,
why does it vary with initial iodine content?
Support from the PRF has enabled our
young group to generate initial results worthy of publication. As previously
reported, this research made grant applications more competitive, with success
in obtaining public and private funds, including an NSF-CAREER award and a
Packard fellowship for science and engineering. This work was a significant
contributor to the awarding of the Exxon-Mobil Solid State Faculty Fellowship
to the PI. The methods developed have been included in multiple additional
proposals, most recently the successful renewal of the Department of Energy
funded Institute for Quantum Matter at Johns Hopkins University. In addition, students
have been exposed to synthetic solid state chemistry techniques and have gained
familiarity with diffraction methods and electrical and magnetic property
measurements, all of which are important skill sets for their future careers. In
short, this funding has allowed us to demonstrate the viability of soft
chemistry methods for preparing metastable solid state materials. Three
publications are expected directly from this work (one published, one under
review, one in preparation), with indirect contributions to two more (both
published).
4O8
only vary slightly with electron count, these results demonstrate that it is
possible to insert barium using organometallic chemistry. More generally, they
offer an exciting possibility: by having the ability to prepare two isostructural
compounds with the same lattice parameters but differing electron counts, it
should be possible to disentangle the effects of electronic changes and
structural changes on the physical properties; this is not normally achievable
due to concomitant change of both in a typical solid solution.
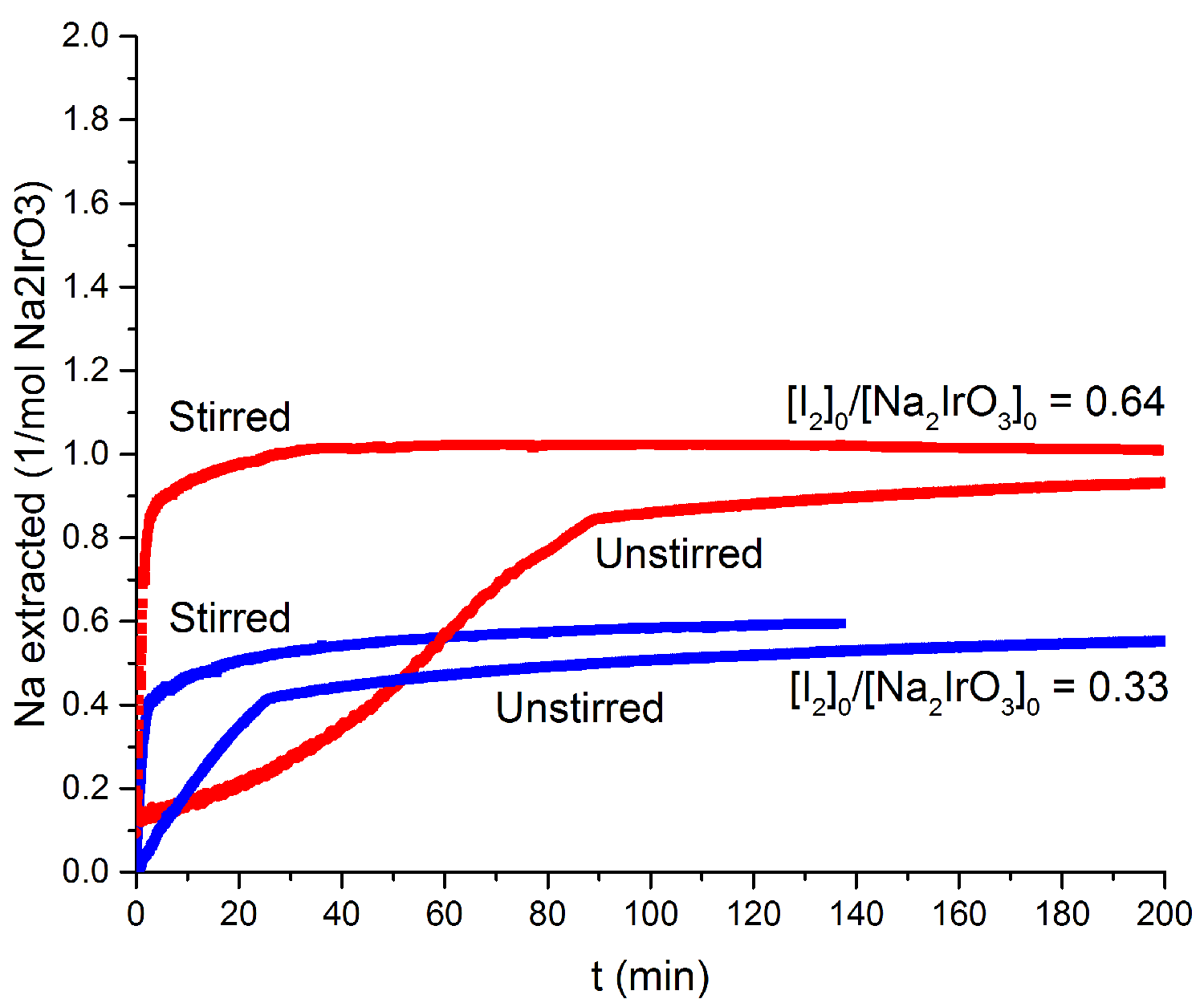
Figure 2: Reaction progress of deintercalation of Na2-xIrO3 for two different initial conditions. There is a pronounced 'kink' in the progress, with or without stirring that changes with initial conditions.
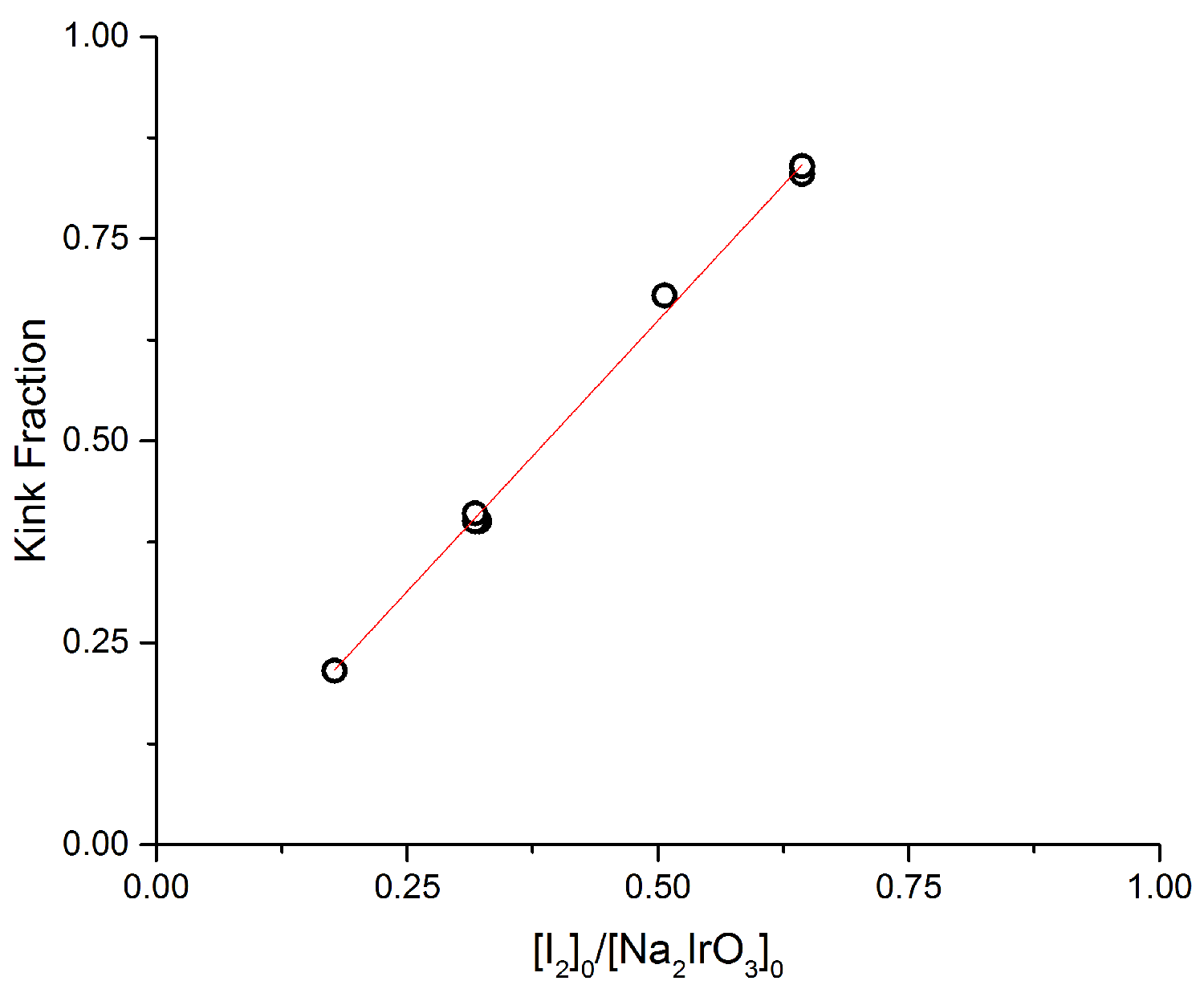
Figure 3: There is a near-perfect linear relationship between the initial concentration of iodide (relative to solid), [I2]0/[Na2IrO3]0, and the value of x in Na2-xIrO3 at which the 'kink' occurs in the kinetics data.











