Reports: UNI751042-UNI7: Fundamental Studies of Swelling Hysteresis in Humidity Sensitive Polymer Films
Adam J. Nolte, PhD, Rose-Hulman Institute of Technology
Introduction
The overarching goal of this work has been to fabricate and investigate humidity swelling hysteresis in thin polymer films. Two polymer film systems were investigated in order to obtain fundamental insights into the types of interactions that cause hysteretic swelling in polymer films.
The first system originally comprised layer-by-layer (LbL) assemblies of poly(allylamine hydrochloride) (PAH) and poly(acrylic acid) (PAA). PAH/PAA LbL films are pH-sensitive, in that the crosslinking density of the films has been shown to be dependent upon the pH of the PAH and PAA solutions during assembly. The second system comprised thin films containing poly(N-isopropylacrylamide) (PNIPAAm). PNIPAAm films are temperature-sensitive, in that the temperature of the film dictates the solvent quality of water via an LCST phenomenon (the LCST is approximately 34 °C); at lower temperatures water is a better solvent for PNIPAAm.
Personnel and Project Timeline
During Summer 2013 two undergraduate chemical engineering students (Z. Yin and Z. Fang) conducted research under the direction of the PI. During the 2013-2014 academic year (and into Summer 2014 for the PI), those students (and the PI) continued their work and data analysis; brief background information and recent results are reported below.
Progress Update on Research Activity 1: “LbL films with controlled crosslink density and secondary interactions”
Background: The primary goal for the first year on Research Activity 1 was to assemble PAH/PAA films at different pH combinations in order to examine how changes in electrostatic crosslinking might affect the humidity swelling hysteresis of the system. As we noted in our previous report, the incremental per-bilayer growth increment of PAH/PAA can change drastically with pH, although this has been primarily observed for dip-coated films. We found that spin-assisted assembly (SA-LbL), however, yielded film growth trends that were remarkably insensitive to the pH conditions of assembly. Additionally, it was found that the humidity swelling curves for all systems were quite similar.
Update: Our initial objective was to investigate how the manner of deposition (dip vs. SA-LbL) affected swelling characteristics, although moving into year 2 of the project we realized that a more fundamental pressing question in light of our year 1 discoveries was determining why deposition method played such an important role in the growth characteristics of the films.
We therefore broadened our investigative scope to include so-called “strong polyelectrolytes” that were relatively pH-insensitive. In particular, we investigated SA-LbL of the PAH/Poly(styrenesulfonate) (PAH/PSS) system, which was known to grow by dip-coating at approximately 1 nm/bilayer. A key discovery this year was that the PAH/PSS system, similar to the fully charged PAH/PAA systems investigated last year, also grew at a much larger growth increment relative to previous dip-coated results. In order to investigate this phenomenon more thoroughly, we purchased a dipper using budgeted funds in order to directly compare dip-coated and SA-LbL films prepared from identical polymer solutions. Future work will focus on comparing not only growth increment (but also internal film chemistry) for both dip-coated and SA-LbL films using reflectometry, swelling hysteresis, and spectroscopic techniques.
Progress Update on Research Activity 2: “Spin-coated, photo-crosslinked temperature sensitive polymer films”
Background: The primary goal for the first year on Research Activity 2 was to obtain a method for reliably fabricating crosslinked thin films of PNIPAAm. We developed a route for synthesizing crosslinked N-isopropylacrylamide films of submicron thicknesses, and showed preliminary data suggesting such films (1) undergo hysteretic swelling, and (2) swell to a greater extent in humid environments at lower temperatures, as would be expected for a system exhibiting LCST behavior.
Update: In the second year, we have done more detailed work to understand the synthesis parameters affecting the degree of swelling change exhibited by a PNIPAAm film due to the LCST effect.
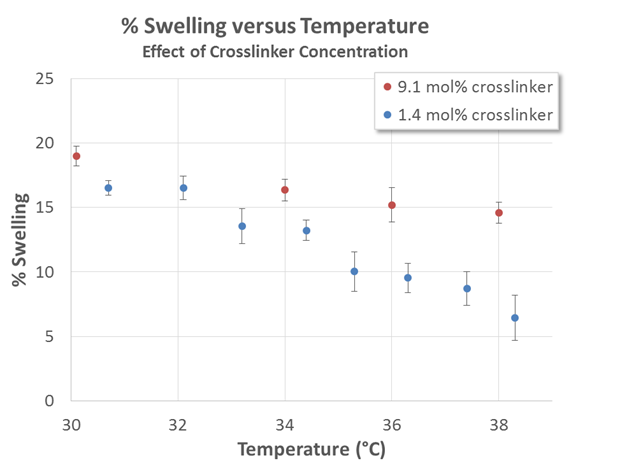
Our first key finding was that increasing concentration of bis-acrylamide crosslinking agent has a detrimental effect on the LCST deswelling effect for films in humid environments subjected to increasing temperatures.
Figure 1 shows experimental results from two PNIPAAm films that were prepared with different concentrations of bis-acrylamide. Note that an increase in crosslinker does not seem to result in any decrease in swelling at low temperatures (on the contrary, we observe slightly higher swelling for the film with a higher crosslinker concentration). As temperature is increased, both films exhibit deswelling consistent with an LCST transition. The film having less crosslinker deswells more than the film with more crosslinker. Separate experimental evidence has suggested that this is due to the fact that the bis-acrylamide is more hydrophilic than the NIPAAm monomer.
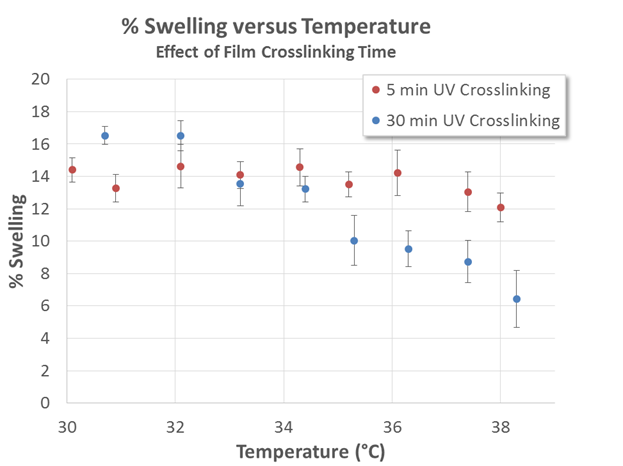
We have also determined the effect of UV polymerization time on the magnitude of the LCST effect. Figure 2 demonstrates that films UV-exposed for only 5 min do not exhibit a significant LCST effect; films exposed for 30 min, however, exhibit LCST-based deswelling upon increasing temperature.
Although films polymerized for only 5 min exhibited humidity-driven swelling, these results confirm that extended exposure to UV is necessary to produce a network capable of sustaining an LCST transition. This is presumably due to the LCST mechanism; increasing polymerization decreases the entropic gains associated with mixing of the water and PNIPAAm. Future work will focus on optimizing the UV exposure time and determining the relation between the LCST effect and the extent of polymerization of the film.
In the final year of the project work, we additionally plan to begin disseminating our work externally as we distill our findings from the research activities into publication form, which will provide additional valuable experience for the participating students.