Reports: DNI653053-DNI6: Direct Characterization of the Structure and Dynamics of Catalytic Monolayers by Coherent Surface-Specific 2D-IR Spectroscopy
Poul B. Petersen, PhD, Cornell University
A large research effort sponsored by the ACS PRF and other funding agencies goes into developing molecular catalysts for CO2 catalysis. In order to make CO2 catalysis practical, a heterogeneous system, where the catalyst is bound to a high surface-area support, is needed to improve the throughput and easily separate the reaction products from the catalytic system. However, when a molecular catalyst is bound to a surface it significantly changes the properties of the catalyst and our current knowledge of how the properties of the catalyst depends on the surface binding is limited. One commonly used platform for immobilizing catalysts is colloidal or nanostructured TiO2. Colloidal TiO2 has the advantage of being a very cheap and high surface-area support that also provide electrical contact but possesses a very large structural heterogeneity. There are a multitude of different ways the catalyst can bind to the surface, which makes it very hard to systematically study how the surface binding affects the properties of the catalyst.
We employ a different approach and use surface-specific spectroscopy in the form of sum-frequency generation (SFG) spectroscopy to study molecular CO2 catalysts bound to single-crystalline TiO2 surfaces. SFG has over the last decades been developed into a powerful method for studying interfaces by measuring their surface vibrational spectra. To probe the properties of surface-bound catalysts, dynamical measurements are necessary and not just their static spectra, but few research groups are capable of performing transient SFG experiments due to their complexity and limited signal strength. Our research effort is to push the limits of transient SFG measurements and use these advances spectroscopic methods to systematically probe the properties of molecular catalysts bound to single-crystalline surfaces as a function of the specific TiO2 surfaces.
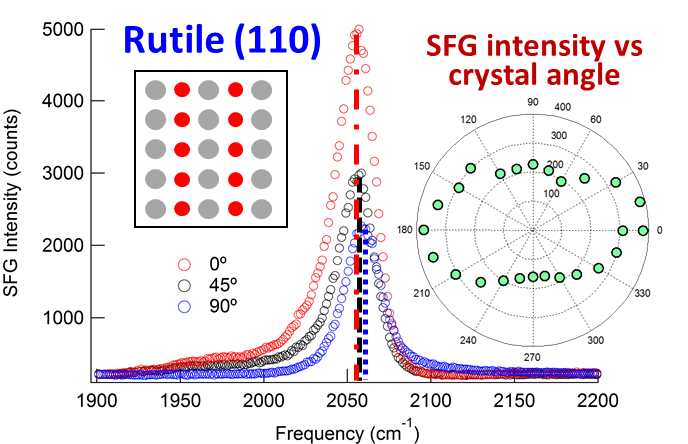
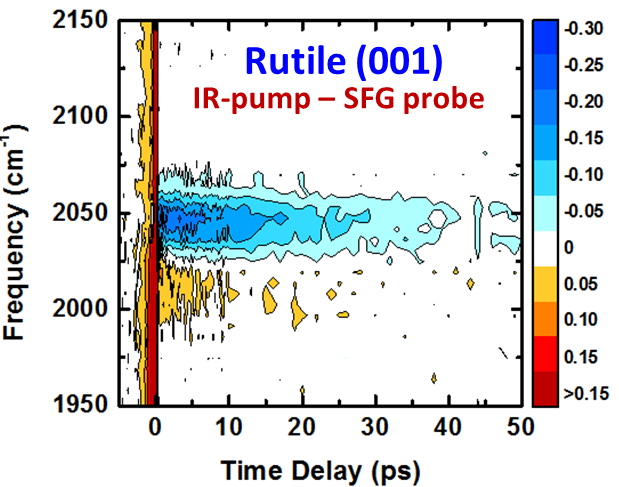
With the ACS PRF grant, we have successfully implemented transient SFG experiments in the form of IR pump – SFG probe experiments to probe the vibrational relaxation of catalyst monolayers on single-crystalline TiO2 surfaces. We have furthermore, optimized the surface-functionalization of catalytic monolayers on single-crystalline TiO2 surfaces, which are remarkable different that colloidal TiO2 surfaces. Our initial efforts have focused on the Re(dcbpy)(CO)3Cl catalyst (ReCO3), where dcbpyis 4,4’-dicarboxy-2,2’bipyridine. We have examined the catalyst on both (110) and (001) single-crystalline rutile TiO2 surfaces. We find that both the structure and the vibrational relaxation dynamics is different at the two surfaces. While rutile (001) exhibit an isotropic catalyst orientation within the surface plane, rutile (110) exhibit C2V symmetry reflecting the underlying symmetry of the rutile surface. This is illustrated in Figure 1, which show the SFG spectra of ReCO3 on rutile (110) as a function of the angle of the crystal with respect to the plane of incident of the laser beams. The vibrational relaxation time is also different at the two surfaces. Figure 2 show the transient IR pump – SFG probe spectra of ReCO3 on rutile (001) as a function of the pump-probe time delay, and Figure 3 compares the vibrational relaxation time at the peak position for rutile (001) and (110). This data prove that that the TiO2 surface structure not only affect the static structure and orientation of the catalyst but also the dynamical properties and thus also likely the catalytic properties. We are currently in the process of writing up these results to be submitted later this fall.
The ACS PRF award have facilitated building up the experimental infrastructure to perform transient SFG experiment, which only a couple of other research groups are capable of. With static and transient SFG experiments, we have successfully shown that both the structure and dynamics of the ReCO3 catalyst depend on the TiO2 surface structure. It is well known that the catalytic properties of metallic surfaces greatly depend on the surface structure and a large research effort goes into discovering the optimal surface structure to drive specific catalytic processes. Our results show that the catalytic properties of molecular catalysts will likely also depend on the TiO2 surface structure and will be important for optimizing chemical transformation of CO2. These initial results and proof of concept experiments was made possible by the ACS PRF award and will be instrumental in obtaining further funding of this project. By providing the experimental infrastructure and the initial results to launch this research direction in my laboratory, the ACS PRF award have had and will continue to have a significant impact on my own scientific career as well as that of my senior graduate student who has been funded on this award.
Figure 1: SFG spectra of ReCO3 on rutile (110) as function of crystal angle with respect to plane of incident.
Figure 2: Vibrational relaxation of ReCO3 on rutile (001) with transient IR pump – SFG probe spectroscopy.
Figure 3: Different vibrational relaxation times of ReCO3 on rutile (110) and (001).