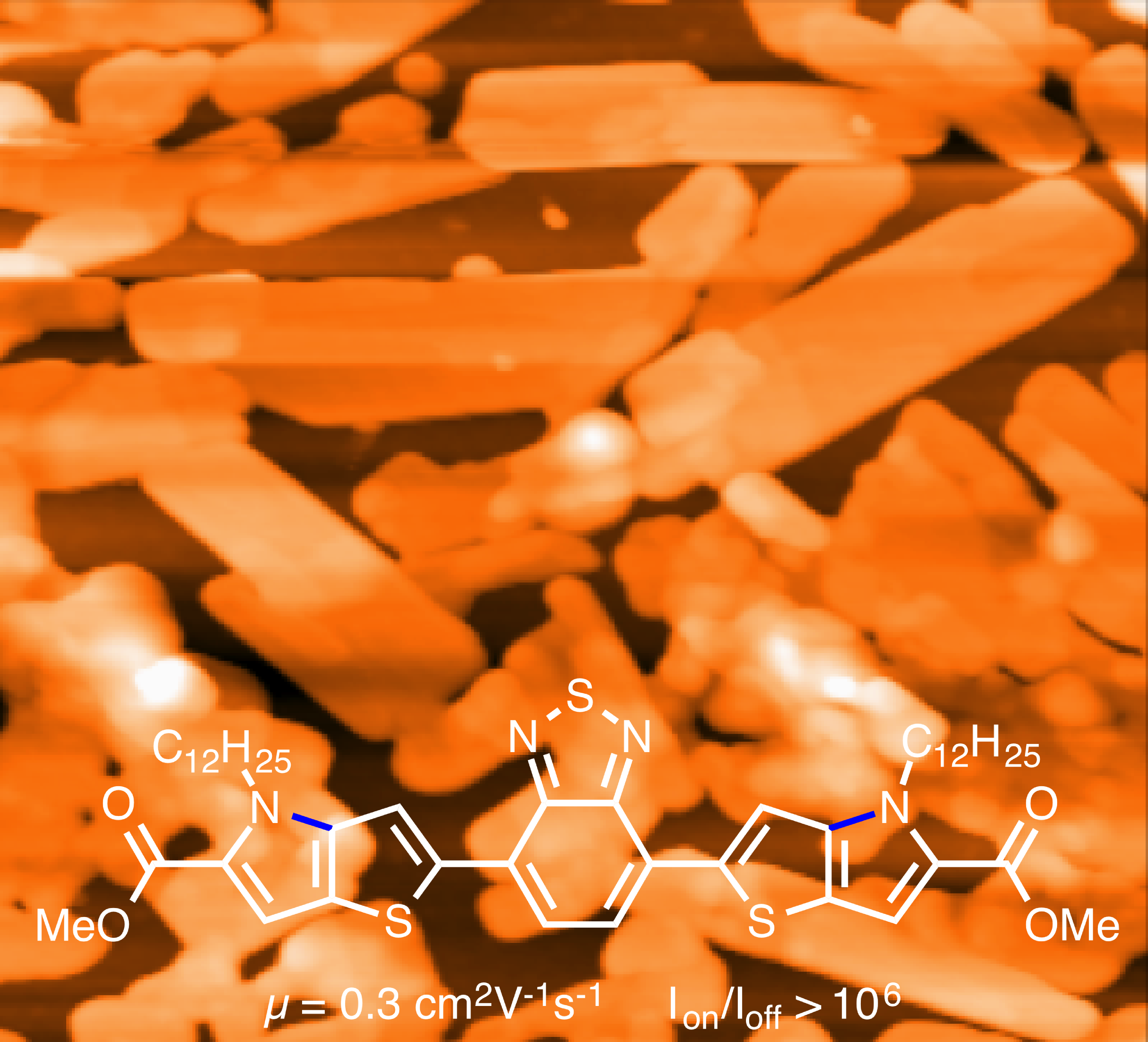
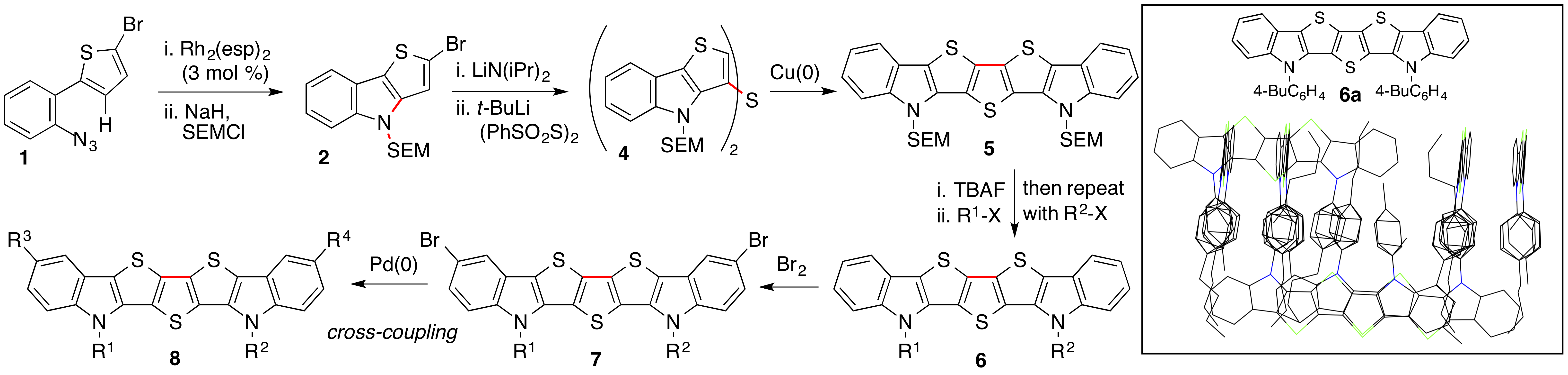
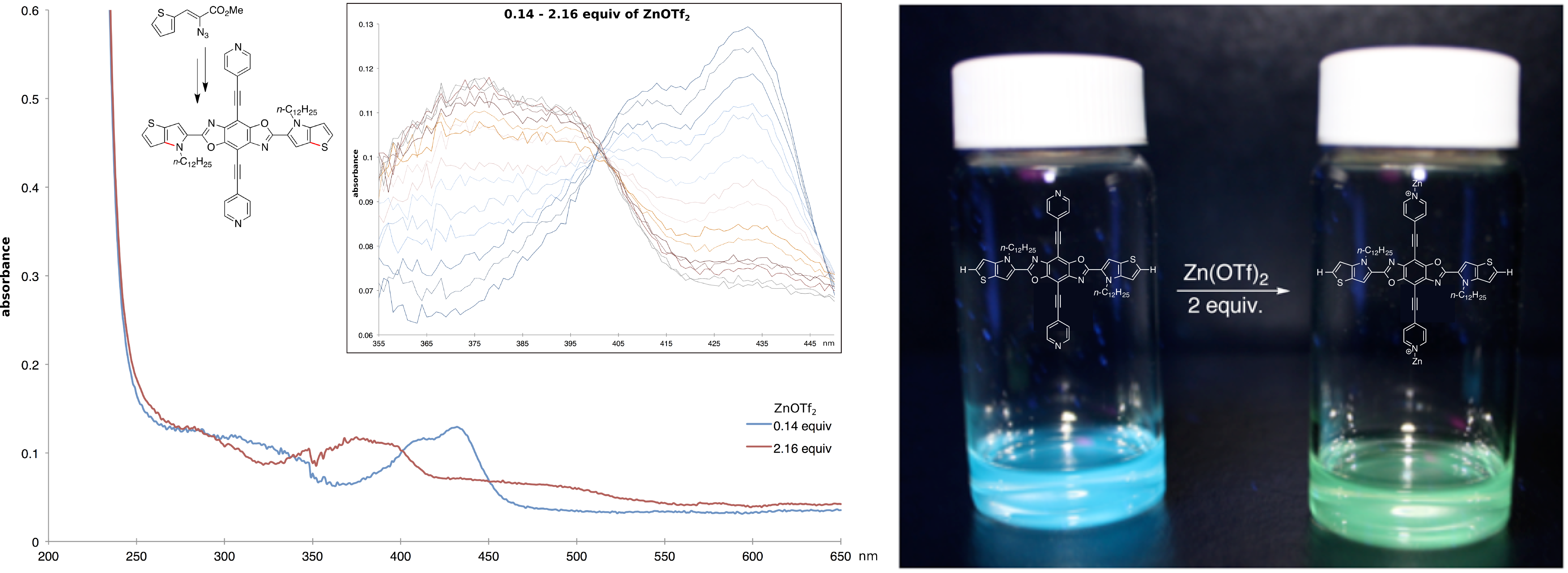
Reports: ND751853-ND7: Synthesis and Design of New N-Heteroaromatic Materials
Tom G. Driver, University of Illinois at Chicago
Interest in semiconducting polymers and fused-oligomeric aromatic molecules for charge transport and
storage/conversion of energy has exploded because of their potential to be
low-cost alternatives to inorganic materials, easy processibility,
and tunable synthesis.