Reports: UNI151900-UNI1: Investigation of the Stabilization of Boron-Containing Cations by Azametallocene Donors: Applications to Reduction, Hydroboration and Borylation Reactions
Timothy J. Brunker, DPhil, Towson University

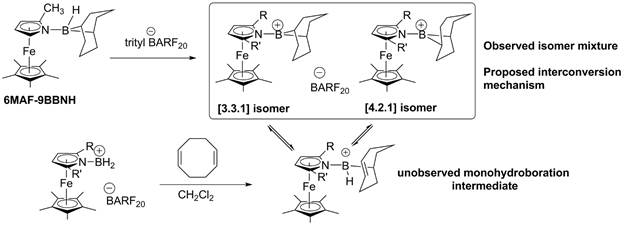
Timothy J. Brunker, DPhil, Towson University


Copyright © American Chemical Society