Reports: DNI753461-DNI7: Phase Behavior of Giant Spherical Amphiphilic Block Copolymers in Solutions
Zhihong Nie, PhD, University of Maryland
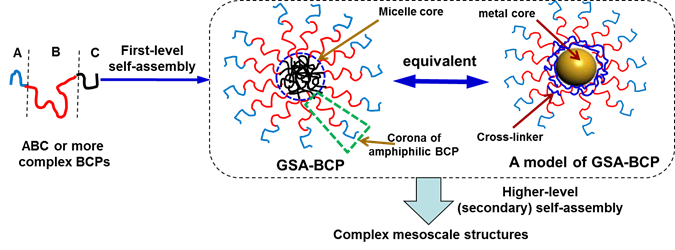
Figure 1. Schematic illustration of the secondary assembly of GSA-BCPs that are produced from the first-level self-assembly of conventional block copolymers (left and middle) and a proposed model for study the assembly mechanism (right).
We have created a model system that enables us to systematically evaluate the secondary self-assembly of micellar architectures into larger superstructures. We used Au nanoparticles (AuNPs) as scaffolds to synthesize building blocks containing a hard core and corona of multiple amphiphilic BCPs (Figure 1). These building blocks mimic the physical and chemical traits of micellar architectures, thus enabling us to simplify and model the structural complexity of polymer micelles. This method is simple yet versatile and does not require multistep synthesis and self-assembly. The model GSA-BCPs allow us to systematically explore the role of thermodynamic (e.g., the length and density of corona BCPs) and kinetic parameters (e.g., hydrodynamics) in the higher-level assembly of micellar architectures. We demonstrated that the assembly of such GSA-BCPs can yield complex hierarchical nanostructures, as a result of their spherical shape and unusual enthalpy contribution of confined polymer chains.
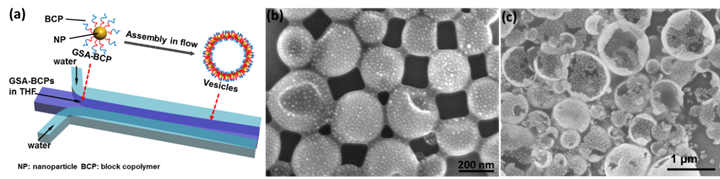
Figure 2. (a) Schematic illustration of the self-assembly of GSA-BCPs into vesicular assemblies in microfluidics. (b,c) Representative SEM images of vesicles assembled from GSA-BCPs containing 5-nm AuNP core (b) and 40-nm Au nanorod core (c) in microfluidics.
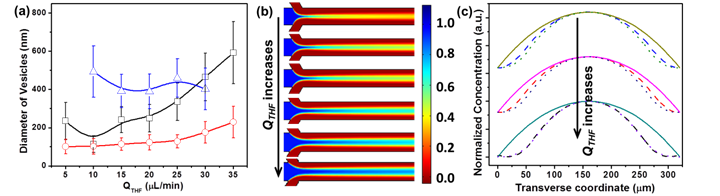
In our paper published on ACS Materials and Interfaces, we reported the effect of hydrodynamic flow on the assembly of such GSA-BCPs into vesicles with a monolayer of GSA-BCPs in the membranes using laminar flows in microfluidic devices (Figure 2). We compared the assembly behaviors of GSA-BCPs with different shapes. Our experimental studies showed that the shape of such GSA-BCPs significantly influences the assembly and packing of the building blocks in the membrane: vesicles with openings were formed for rod-like GS-BCPs at certain hydrodynamic condition, while small spherical GSA-BCPs prefer to assemble into vesicles without defects. Moreover, the competitive diffusion of solvent/GSA-BCPs along the transverse direction of the microchannel plays an important role in the assembly process (Figure 3). The diffusion coefficient of a species (i.e., solvent molecules and GSA-BCPs) is inversely proportional to their size. For molecular amphiphiles, their size and diffusion rate are generally on the same order of magnitude as the solvent molecules. In this case, there is no clear boundary of the concentration gradient of solvents and amphiphiles. In contrast, the significant increase in the dimension of GSA-BCPs drastically decreases their diffusion rate. The delay in the transverse diffusion of GSA-BCPs leads to a significant difference in the gradients of solvents and GSA-BCPs (Figure 3c). This phenomenon strongly affects the kinetic process of GSA-BCP self-assembly, as reflected by the big difference in the dimension of assembled structures from GSA-BCPs and conventional BCPs (Figure 3a).
Figure 3. (a) The diameter of vesicles assembled from pure BCPs (○), GSA-BCPs with 5 nm-AuNP core (□) and 20 nm-Au core (Δ) as a function of the THF flow rate (QTHF). (b) Simulated GSA-BCPs concentration distribution at various QTHF. (c) Simulated concentration profiles of pure BCPs (solid line), GSA-BCPs with 5 nm-AuNP core (dash line) and 20 nm-AuNP core (dot line) as a function of the transverse coordinate of the microchannel.
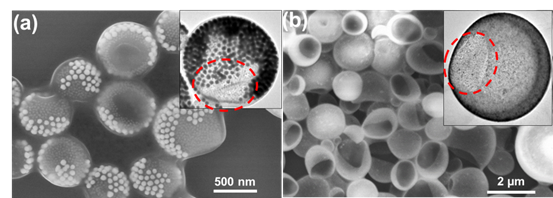
We are currently exploiting the intrinsic thermodynamic tendency of the phase separation behaviors of GSA-BCPs, in order to gain new understanding of the phase behaviors of GSA-BCPs. We synthesized a series of GSA-BCP subunits in which we systematically vary: the size of "micelle" cores, the length and chemical composition of each block of corona BCPs, and the density of corona BCPs. Three systems that are currently ongoing include: a mixture of binary GSA-BCPs carrying demixing corona BCPs, a mixture of GSA-BCPs carrying miscible corona BCPs, and a mixture of GSA-BCPs and conventional BCPs. Our preliminary results showed that resembling molecular BCPs, the GSA-BCPs can internally micro- or macro-phase separate at a larger length scale within discrete aggregates, driven by enthalpy and/or entropy (Figure 4).
Figure 4. Phase-separated Janus-like vesicles assembled from GSA-BCPs: (a) a mixture of GSA-BCPs (bright), and conventional BCPs and magnetic NPs (grey), and (b) a mixture of GSA-BCPs (black) and conventional BCPs (grey). The red dashed circles indicate magnetic NP domain (a) and polymer domain (b).
The research program involves three postdoctoral researchers, two graduate students (Yijing Liu and Shaoyi Zhang), one undergraduate student (Yunlong Yang), and one assistant professor (Zhihong Nie). The results generated in this work constituent a significant portion of the PhD thesis of Yijing Liu who is expected to graduate in the summer of 2015. The undergraduate student is one of the co-authors of one publication, reflecting the active involvement and contribution of undergraduate researcher in this work. Moreover, the knowledge obtained from this work also leads to us to the development of excitingly new research directions, such as on the design and assembly of supracolloidal molecules.