Reports: DNI752275-DNI7: Synthesis and Structure-Property Relationship Studies of Polymers Containing Core-Substituted Naphthalene Diimides
Genevieve Sauve, PhD, Case Western Reserve University
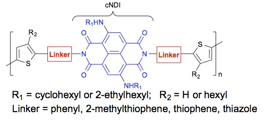
Figure 1. Proposed alternating copolymers.
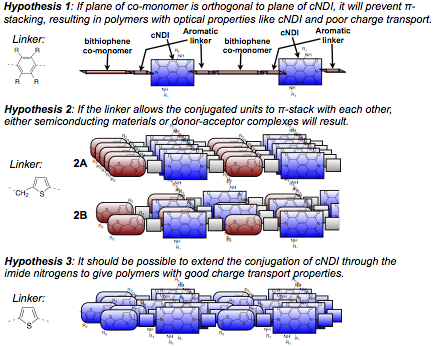
Figure 2. Linkers and hypothesis tested in this proposal.
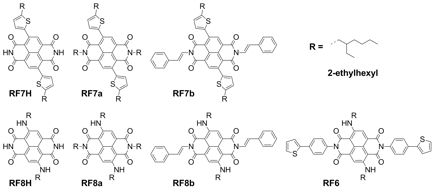
During our first year, we reported efforts to test the first two hypotheses. During this second year, we focused our efforts in testing the third hypothesis about extending conjugation of cNDI through the imide nitrogens. Installing a conjugated moiety at the imide position that can lay in the same plane as cNDI turned out to be very challenging. Usually, the aryl imide group is out of plane with the cNDI core due to steric hindrance. We were unable to install a thiophene at the imide position as originally planned due to synthetic obsticles. We found an alternative imide group that would allow us to test our hypothesis: a styryl group. We were able to install the styryl group at the imide positions using Chan-Lam chemistry and optimized the reaction conditions. Unfortunately, we only obtained low yields, which prevented us from making polymers. Instead, we synthesized a series of molecules to study the effect of styryl imide substitution on optical and electrochemical properties of cNDI, shown in Figure 3.
Figure 3. Six core-substituted NDI molecules with different imide substituents studied.
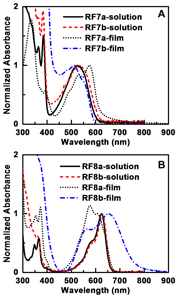
2-Ethylhexylamino and 5-(2-ethylhexyl)thiophene groups were used as core substituents. The optical and electrochemical properties of styryl imide substituted compounds were compared with other imide substitutions including hydrogen, 2-ethylhexyl, and 4-thienylphenyl. Generally, the imide substituents had little effect on the optical properties, except when the combination of alkylamino core and styryl imide substituents was used (Figure 4). In this latter case, we observed a 104 nm red-shift of the absorption onset upon film formation, resulting in an unusually broad visible absorption (500-800 nm) for these types of molecules. This is explained by the planarity of the molecule, which allow for pi-stacking in film and the formation of intermolecular donor-acceptor type interactions. We think it is unlikely that conjugation is extended to the styryl groups because the solution spectra is unaffected. Nevertheless, the styryl imide substituents can accept electrons from the imide nitrogen through resonance effects, and was found to stabilize both the anion and dianion. The stabilizing effect on the dianion were stronger for the styryl imide groups than for 4-thienylphenyl imide groups, indicating that planarity between the NDI core and imide substituent has an impact on the electrochemical properties.
Figure 4. Comparison of solution and thin film absorption spectra of A) RF7a and RF7b; B) RF8a and RF8b. The spectra were normalized, setting the low energy absorption band to 1. This shows that RF8b had an unusually broad thin-film absorption spectra in the visible range (500 -800 nm), due to its planar geometry that allows it to pi-stack well in films.
While working with cNDI molecules, we became interested in further tuning the opto-electronic properties of cNDI by sulfur substitution of the carbonyls. Thionation of the carbonyls had been reported in a patent for NDI molecules, but mixtures of isomers were obtained that could not be isolated easily. Here, we were able to selectively convert imides to monothiomides using Lawesson's reagent, shown in scheme 1. Analysis by NMR and x-ray crystallography showed that the thionation occurred at the sulfur atoms located distally to the core amine. The selectivity appears to be sterically controlled by a combination of the core and imide substituents. Whithout these substituents, mixtures of isomers and higher substitution were obtained.
Scheme 1. Synthesis of thionated cNDI derivatives.
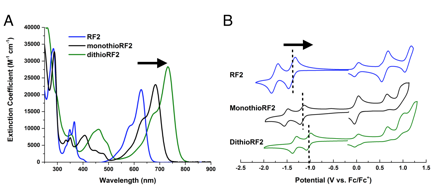
Figure 5 shows the absorption spectra and cyclic voltammograms obtained for RF2, MonothioRF2 and DithioRF2. The UV-visible absorption spectra exhibit a 50 nm shift for each sulfur substitution: 630, 680, 730 nm for RF2, RF2-1S and RF2-2S, respectively. Cyclic voltammetry showed lower reduction potentials (-1.39, -1.17 and -1.03 V vs Fc/Fc+) and decreasing HOMO-LUMO gaps (2.00, 1.83 and 1.71 eV) for RF2, RF2-1S and RF2-2S, respectively. Thionation of the carbonyls is a promising tool to develop new materials that combine high electron affinity and absorption in the near IR.
Figure 5. A) UV-visible absorption spectra and B) cyclic voltammograms showing the effect of thionation on the optical and electrochemical properties of a cNDI molecule.
We are also working towards making thin-film transistors to evaluate the charge transport properties of these novel cNDI-based molecules. We expect interesting charge transport properties for both groups of cNDI molecules reported here: the styryl-modified NDI molecules may have improved charge transport due to their planar geometry, and the thionated cNDI molecules may have improved charge transport due to the larger sulfur molecular orbitals. We are setting up the measurement tools and will be testing our molecules soon.
This ACS-PRF supported graduate student Roshan Fernando and Forrest Etheridge. Roshan Fernando successfully defended his thesis and received his Ph.D. end of August, 2014. He is now a postdoctoral fellow in my group. This grant has allowed us to learn about the chemistry and properties of cNDI, which we are using for writing a grant aimed at discovering new molecules with unprecedented opto-electronic properties and to enrich our understanding of functional materials. This grant partially funded research that resulted in one publication during the first year and will result in two more publications: one currently under review for New J. Chem. and another currently in preparation for J. Org. Chem.