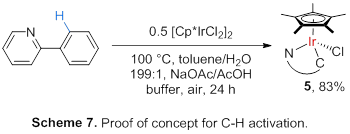Reports: DNI352119-DNI3: Acylation of Arenes via Catalytic C-H Bond Functionalization and Aerobic Alcohol Oxidation
Marion H. Emmert, PhD, Worcester Polytechnic Institute
1. OVERVIEW
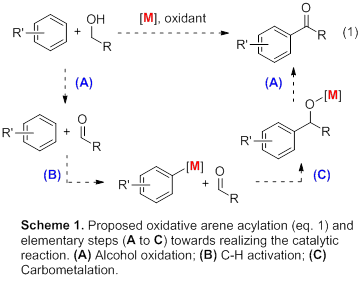
In order to create sustainable syntheses for arylketones [[1]], we have proposed to investigate a novel catalytic cycle (Scheme 1), consisting of (A) aerobic alcohol oxidations, (B) C-H activation, and (C) aldehyde carbometalation.
2. SCIENTIFIC REPORT
2.1 Ir catalyzed aerobic alcohol oxidations.
2.1.2. Non-innocence of Ag. Further investigations of aerobic alcohol oxidation suggested that the presence of Ag(I) lessens the catalytic performance. Possible mechanisms for this process are Ag promoted oxidations of the Cp*Ir catalysts [[2]] or the formation of dinuclear Ir species from Ir-H intermediates in the presence of Ag. [[3]]
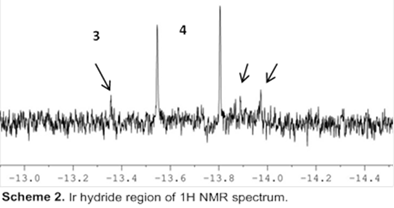
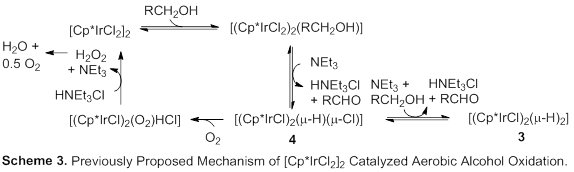
2.1.3 Isolation of decomposition products. In order to better understand which catalyst decomposition products are formed during catalysis, we investigated the inorganic complexes after the reaction and observed a catalytically inactive, bimetallic Ir hydride species [Cp*IrCl(µ‑H)]2 (3) at ‑13.6 ppm and [(Cp*IrCl2)2(µ‑H)(µ‑Cl)] (4) (Scheme 2) [[4]].
This suggests that several different Ir-H species might be involved during the air oxidation of alcohols or may be formed as decomposition products. No Ir-H resonances were in the presence of Ag additives.
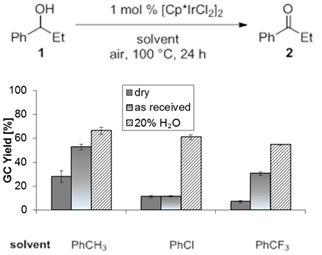
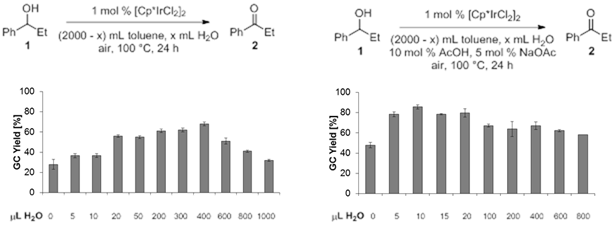
2.1.4 Effect of solvents and additives on catalyst stability. The effect of H2O and other additives was investigated, suggesting that the water content indeed contributes to catalyst stabilization (Schemes 4/5).
We speculated that the catalytic
cycle would be accelerated by a proton buffer. Consequently, AcOH/NaOAc and CF3CO2H/NaO2CCF3
buffer systems were tested in various toluene/H2O mixtures. In all
cases, the AcOH/NaOAc buffer provided higher 24 h yields of 2 up to 83%.
Reactions were also performed at 0.1 mol % catalyst loading, affording 270 TONs
[[5]].
This is to our knowledge the highest TON achieved to date for Ir catalyzed
aerobic alcohol oxidations, which do not proceed through acceptor-less
dehydrogenation mechanisms [[6]].
2.1.5 Substrate scope study.
A variety of secondary alcohols were readily transformed into the resulting
ketones. Overall, benzylic secondary alcohols were reactive substrates with
both electron-rich and electron-neutral substituents; secondary, non-benzylic
alcohols were also good substrates.
2.2 Aldehyde carbometalations.
Efforts during
2013/14 focused then on optimizing the yields of aldehyde carbometalations for
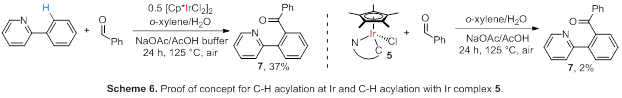
the stoichiometric reactions at Ir, since the respective yields for this
reaction (Scheme 6) was determined to produce less than 0.5% of aryl ketone
product.
We demonstrated
that the proposed C-C bond formation proceeds under conditions analogous to the
best aerobic oxidation conditions by reacting [Cp*IrCl2]2
with 2-phenyl pyridine in the presence of benzaldehyde and NaOAc/AcOH buffer
(Scheme 6). Reactions between cyclometalated complex 5 and benzaldehyde
under analogous conditions proceeded less readily (Scheme 6) and afforded only
2% of the acylation product 7.
2.3 C-H
Activation.
2.3.1 C-H
Activation under Aerobic Oxidation Conditions. We sought
to demonstrate that C-H activation is possible under aerobic oxidation
conditions (Scheme 7); formation of the cyclometalated complex 5 was
observed.
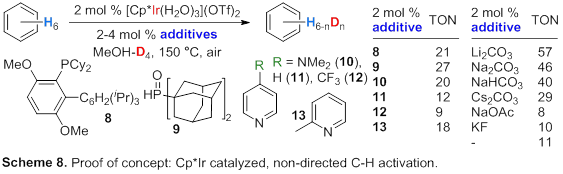
2.3.2 Non-Directed C-H Activation
with Oxidatively Stable Ligands and Additives.
We synthesized Cp*Ir(H2O)3(OTf)2
as a Ag-free catalyst precursor for H/D exchange; 11 TONs were obtained.
Several ligands and basic additives (Scheme 8) further promoted the C-H activation
reactivity (TONs >20). We conclude that ligands with moderate steric
hindrance and strong electron-donating features are most promising for
non-directed C-H activation.
3. IMPACT OF AWARD ON PI’S CAREER
AND ON SUPPORTED STUDENTS
A publication on aerobic oxidation
is currently under review. In addition, a current senior thesis student
explores aerobic oxidations of biomass substrates. Another senior thesis
student continues the work in the PIs laboratory on H/D exchange.
The work completed under this award has
provided data for 3 poster presentations by the PI (2 at Gordon Research
Conferences; 1 at WPI), 2 poster presentations by the postdoc, and 3 poster
presentations by an undergraduate researcher in the group. The PI has spoken
about the work conducted under this grant in 4 invited lectures. The results
have been used to apply for NSF funding in 2013 and 2014.
Scheme 4. Effect of Water in Different Solvents.
Scheme 5. 24 h Reactivity vs. water
content without (left) and with buffer system (right).
[1] Bauer, K.; Garbe, D.; Surburg, H., Common fragrance and flavor materials. 4th Edition ed.; Wiley-VCH: Weinheim, 2001.
[2] Ringenberg, M. R.; Kokatam, S. L.; Heiden, Z. M.; Rauchfuss, T. B., J. Am. Chem. Soc. 2007, 130 (3), 788-789.
[3] a) Turlington, C. R.; Harrison, D. P.; White, P. S.; Brookhart, M.; Templeton, J. L., Inorg. Chem. 2013, 52 (19), 11351-11360; b) Feldman, J.; Calabrese, J. C., Inorg. Chem. 1994, 33 (25), 5955-5956; c) Bachechi, F., J. Organomet. Chem. 1994, 474 (1–2), 191-197; c) Einstein, F. W. B.; Jones, R. H.; Zhang, X.; Sutton, D., Can. J. Chem. 1989, 67 (11), 1832-1836; e) Sykes, A.; Mann, K. R., J. Am. Chem. Soc. 1988, 110 (24), 8252-8253.
[4] Feng, Y.; Jiang, B.; Boyle, P. A.; Ison, E. A., Organometallics 2010, 29 (13), 2857-2867.
[5] TONs were calculated in agreement with reference 14 based on the amount of [Cp*IrCl2]2 in the reaction.
[6] The so far highest TON in aerobic, non-dehydrogenative alcohol oxidations at Ir is 70 with [Cp*IrCl(bipyrimidine)]+ as catalyst, as reported in A. Gabrielsson, P. van Leeuwen, W. Kaim, Chem. Commun. 2006, 4926-4927. However, the catalyst showed fast decomposition under the reported reaction conditions.