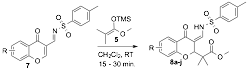Reports: UR153237-UR1: Investigation of a Stereoselective Tandem Inverse-Demand Hetero-Diels-Alder/Tin-Free Radical Process for the Synthesis of Highly Substituted Heterocycles
Jake R. Zimmerman, PhD, Ohio Northern University
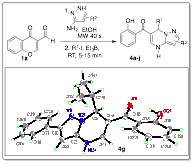
My group spent the first half of this grant period working on a project that involved synthesizing a new class of dihydropyrazolopyrimidines. This class of compounds represents an important group of heterocycles due to their synthetic utility, significant biological activities and pharmacological importance as purine analogs.[1] Table 1 highlights a brief summary of this work, which was published in early 2014.[2]
Table 1. Radical and pyrazole scope of substituted dihydropyrazolo-pyrimidine formation.
Entry |
Product |
R1 |
R2 |
Yield (%)a |
1 |
4a |
iso-propyl |
5-CH3 |
85 |
2 |
4b |
tert-butyl |
5-CH3 |
93 |
3 |
4c |
c-hexyl |
5-CH3 |
80 |
4 |
4d |
c-pentyl |
5-CH3 |
70 |
5 |
4e |
ethyl |
5-CH3 |
72 |
6 |
4f |
iso-propyl |
5-H |
78 |
7 |
4g |
iso-propyl |
5-Ph |
82 |
8 |
4h |
iso-propyl |
4-CN |
85 |
9 |
4i |
iso-propyl |
4-CO2Et |
70 |
10 |
4j |
iso-propyl |
4-Br-5-CH3 |
73 |
a Isolated yields.
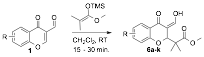
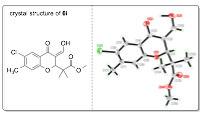
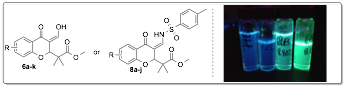
Currently my group is investigating the preparation of a new class of highly fluorescent chromone derivatives. We were interested in an inverse-demand hetero-Diels-Alder (IDHDA) reaction using silylenol ethers and 3-formylchromones. After studying this reaction for several months it was discovered that reacting enol ether 5 with a variety of formylchromones yielded conjugated enol products 6 (see Table 2). Furthermore, we found that simple irraditation of these compounds using a basic long-wave UV lamp (~365 nm) resulted blue fluorescence. There are a variety of commercially available 3-formylchromones and, therefore, we synthesized a small library of these enol products in order to study their fluorescent properties.
Table 2. Synthesis of fluorescent enol chromone derivatives.
Entry |
R |
Product |
Yield (%)a |
1 |
6-H |
6a |
77 |
2 |
6-CH3 |
6b |
62 |
3 |
6-OCH3 |
6c |
62 |
4 |
6-CH2CH3 |
6d |
91 |
5 |
6-F |
6f |
87 |
6 |
6-Cl |
6g |
66 |
7 |
6-Br |
6h |
63 |
8 |
6-Cl, 7-CH3 |
6i |
77 |
9 |
6,8-Cl |
6j |
71 |
10 |
6,8-Br |
6k |
54 |
aIsolated Next, we focused on synthesizing compounds 7 which were easily prepared by condensing a variety of sulfonamides with 3-formylchromones. These sulfonamide derived starting materials also underwent fast IDHDA reactions with silylenol ether 5 (see Table 3). The resulting enamine products 8 gave intense green fluorescence under long-wave UV irradiation (~365 nm).
Table 3. Synthesis of fluorescent enamine chromones derivatives.
Entry
R
Product
Yield (%)a
1
6-H
8a
78
2
6-CH3
8b
80
3a
6-H
8c
72b
4a
6-CH3
8d
52b
5
6-CH2CH3
8e
70
6
6-OCH3
8f
97
7
6-F
8g
90
8
6-Cl
8h
80
9
6-Br
8i
88
10
6,8-Cl
8j
73
a Isolated yield. b p-methoxy substitution on sulfonamide aryl ring. With a series of new fluorophore compounds in hand, absorption and emission maxima along with quantum yields were measured in methylene chloride (Table 4). The enol compounds (6) gave emission maxima from ~450-485 nm. The quantum yields for the enol derivatives were moderate ranging from 2-20%. The enamine products gave significantly better quantum yields, especially when an electron-donating group is located on the chromone aryl ring (see entries 6, 10, 11 and 14).
Table 4. Absorption, emission and quantum yields for newly synthesized fluorophores.
Entry
Product
λabsa nm
λemb nm
Stoke Shift
Φc (%)
1
6a
362
446
84
7
2
6c
383
482
99
20
3
6f
364
486
122
2
4
6l
371
451
80
3
5
8a
358
479
121
37
6
8b
367
492
125
60
7
8b
380
468
88
8d
8
8b
364
479
115
27e
9
8c
358
479
121
28
10
8d
367
492
125
67
11
8e
370
490
120
64
12
8e
369
496
127
16d
13
8e
364
481
117
30e
14
8f
390
529
139
62
15
8g
367
492
125
33
16
8h
367
483
116
39
17
8i
343
483
140
34
18
8j
355
486
131
14
19
8k
368
489
121
21
a Absorption maximum. b Emission maximum. c Fluorescent quantum yield in CH2Cl2. d Fluorescent quantum yield in CH3CN. e Fluorescent quantum yield in cyclohexane. In conclusion, we will continue to investigate the synthesis of these new fluorescent chromones. There are several sites on the starting materials that can be varied in order to tune the spectroscopic properties of these products.
[1]. (a) Bhat, G.A; Montero, J. G.; Panzica, R. P.; Wotring, L. L.; Townsend, L. B. J. Med. Chem., 1981, 24, 1165-1172; (b) Petrie, C. R.; H. B. Cottam, H. B.; McKernan, P. A.; Robins, R. K.; Revankar, G. R. J. Med. Chem., 1985, 28, 1010-1016; (c) Zacharie, B.; Connolly, T. P.; Rej, R.; Attardo, G.; Penney, C. L. Tetrahedron, 1996, 52, 2271-2278; (d) Parker, W. B.; Secrist, J. A.; Waud, W. R. Curr. Opin. Investig. Drugs, 2004, 5, 592-602; (e) Engers, D. W.; Frist, A. Y.; Lindsley, C. W.; Hong, C. C.; Hopkins, C. R. Bioorg. Med. Chem. Lett., 2013, 23, 3248-3252; (f) Hanan, E. J.; Abbema, A.; Barrett, K.; Blair, W. S.; Blaney, J.; Chang, C.; Eigenbrot, C.; Flynn, S.; Gibbons, P.; Hurley, C. A.; Kenny, J. R.; Kulagowski, J.; Lee, L.; Magnuson, S. R.; Morris, C.; Murray, J.; Pastor, R. M.; Rawson, T.; Siu, M.; Ultsch, M.; Zhou, A.; Sampath, D.; Lyssikatos, J. P. J. Med. Chem., 2012, 55, 10090-10107.
[2]. Zimmerman, J.; Myers, B.; Bouhall, S.; McCarthy, A.; Johntony, O; Manpadi, M. Tetrahedron Lett. 2014, 55, 936-940.