Reports: ND651290-ND6: Exploring Electronic-Transfer Pathways of Hot Electrons in Organically-Assisted Metal Catalysts
James P. Lewis, Ph.D., West Virginia University
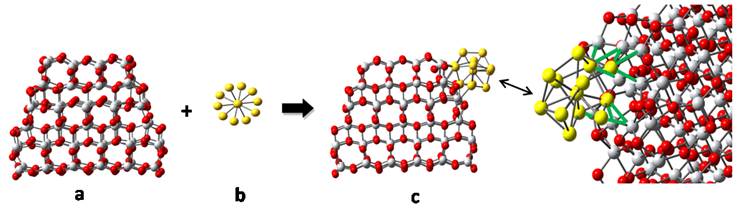
James P. Lewis, Ph.D., West Virginia University

Copyright © American Chemical Society