Reports: DNI451842-DNI4: Hydrazone-Based Rotary Switches as Proton-Relay Systems
Ivan Aprahamian, Dartmouth College
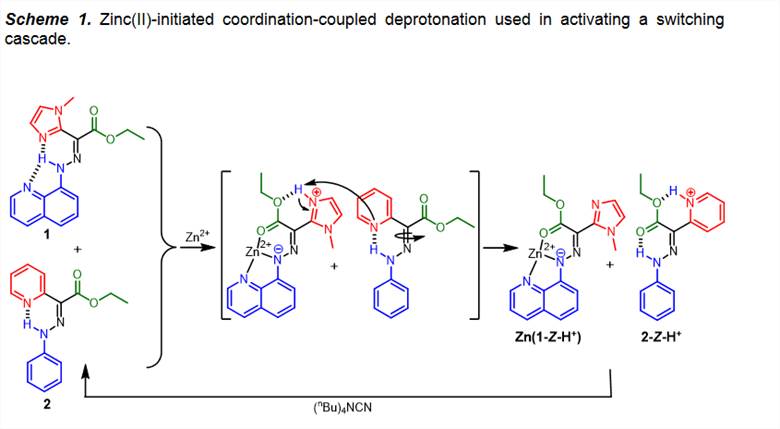
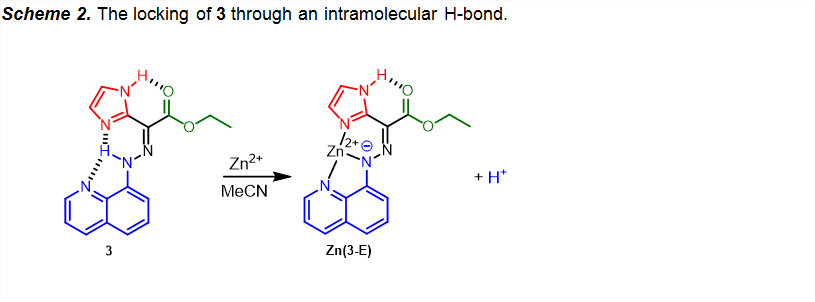
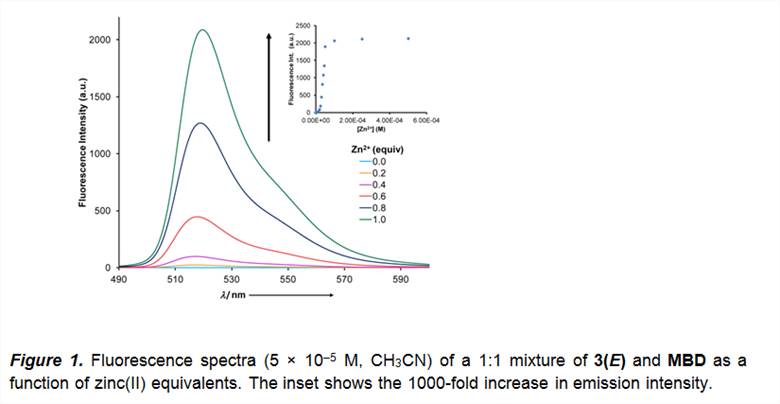
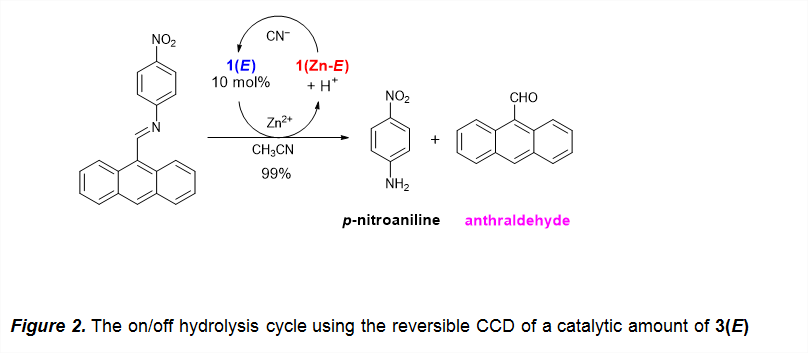
Nonetheless, coordination leads to deprotonation,
Ivan Aprahamian, Dartmouth College

Nonetheless, coordination leads to deprotonation,



Copyright © American Chemical Society