Reports: UR350700-UR3: The Synthesis and Characterization of Early Transition-Metal Complexes with Terminal Nitrido Ligands
Colin D. Abernethy, PhD, Sarah Lawrence College
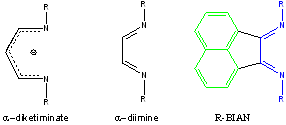
In recent years, Mindiola et al. have prepared and studied the reactivity of a series of vanadium nitride complexes supported by a “nac-nac” (α-diketimiinate, Figure 1) co-ligand. In order to further advance our understanding of the highly reactive V≡N moiety, we sought to develop the coordination chemistry of a structurally related, didentate N-donor ligand, 1,2-bis[(2,6-diisopropylphenyl)imino]acenaphthene (dpp-BIAN, Figure 1), with vanadium with a view to assess its suitability as a coligand for the syntheses of new vanadium-nitride species. R-BIAN ligands may be regarded as α-diimines that are fused to a naphthalene unit (Figure 1). This fusion serves to protect the α-diimine moiety from nucleophilic attack, thus making R-BIAN ligands much more robust and, in turn, conferring much greater stability to their complexes.
Figure 1
During the past 12 months we have investigated the reactivity of dpp-BIAN towards a number of vanadium chloride compounds in order to prepare a dpp-BIAN complex of vanadium that can serve as a starting material for the synthesis of a new family of (dpp-BIAN)vanadium nitride complexes.
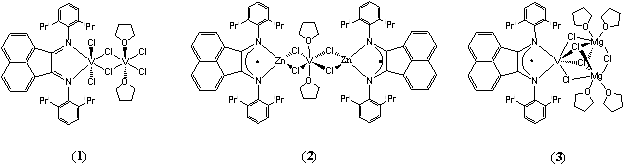
The reaction of dpp-BIAN and VCl3(thf)3 in THF affords (dpp-BIAN)VCl2(μ2-Cl)VCl2(thf)2 (Figure 2, 1), a paramagnetic chloride-bridged dinuclear vanadium(III) complex with a neutral dpp-BIAN ligand coordinated to one of the V(III) ions. In contrast, reaction of dpp-BIAN with the vanadium(II)-containing species,[V2(μ2-Cl3)(thf)6]2[Zn2Cl4(μ2-Cl)2], in THF at room temperature produces the linear trinuclear, bimetallic complex [(dpp-BIAN)Zn(μ2-Cl)2]2V(thf)2 (Figure 2, 2), a paramagnetic vanadium(II)-zinc species with radical anionic dpp-BIAN ligands coordinated to the zinc ions. We have also discovered that the reaction between equimolar amounts of dpp-BIAN, VCl3(thf)3, and Mg in THF gives (dpp-BIAN)V(μ2-Cl)3(μ3-Cl)2Mg2(thf)4 (Figure2, 3), a triangular trinuclear vanadium(II)-magnesium paramagnetic species with a radical anionic dpp-BIAN coordinated to the vanadium(II) ion.
Figure 2
The successful synthesis of 3 is particularly exciting as vanadium(II) complexes supported by N-donor ligands are powerful reducing reagents capable of binding a variety of substrate species and effecting two- or three-electron reductions. We have, therefore, begun to study the reactivity of this species towards reducible nitrogen-containing substrate molecules.
Undergraduate students will present accounts of these findings at the Southeastern Regional Meeting of the American Chemical Society at Nashville, TN in October 2014 and at the National Meeting of the American Chemical Society at Boston, MA in August 2015.
Three of the students who have been involved in the conduct of this research are applying for admission to chemistry graduate programs this year. These students will be the first-ever graduates of Sarah Lawrence College to study for advanced degrees in chemistry.