Reports: ND152040-ND1: The Generation of Acylnitrenes Via Oxidation of Nitriles
Liming Zhang, University of California (Santa Barbara)
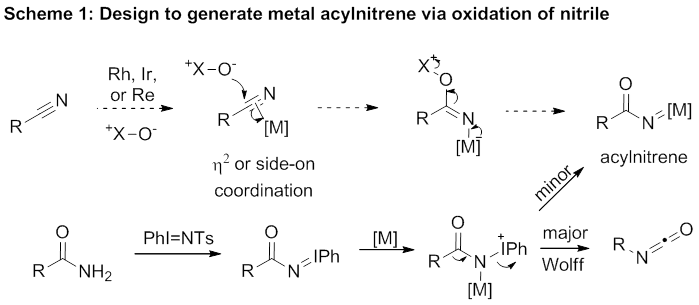 |
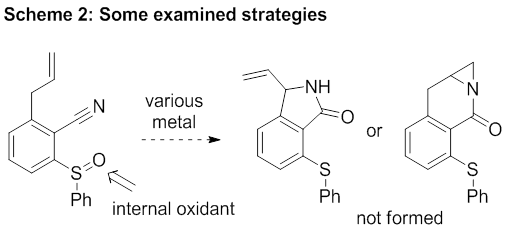
For nearly a year Dr. Guojun Pan was supported by this grant and focused on exploiting this design. This ambitious and rarely precedent strategy was highly challenging and proven to be difficult to implement. For example, we tried intramolecular strategies (Scheme 2) to facilitate the oxidation by tethering an oxidant to the cyano group. Unfortunately, no desired product was detected. Other strategies to force the requisite
 |
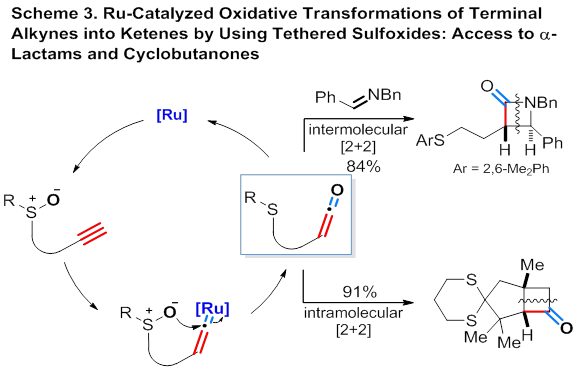
At the meanwhile, consistent with the idea of new direction, this grant partially funded a new project, which concerned oxidation of alkynes into ketenes via Ru catalysis and was hence different from our continuing homogeneous gold catalysis. As shown in Scheme 3, oxidation of Ru vinylidenes, in situ-generated from terminal alkynes and a Ru catalyst, into ketenes is realized using tethered mild sulfoxides. The net result is a Ru-catalyzed oxidative transformation of terminal alkynes into highly valuable ketenes. Moreover, the ketenes generated in this paper are demonstrated to undergo the characteristic ketene [2+2] cycloaddition reactions with tether alkenes and external imines, yielding synthetically versatile bicyclic cyclobutanones and β-lactams, respectively. Although Ru vinylidene has been extensively studied, this novel oxidation opens a new approach to further exploring its synthetic utility.
 |
In conclusion, although we have not been able to generate metal acylnitrene intermediates and study their synthetic utility, this ND grant provides me valuable opportunities to explore new research topics and my graduate students and postdoctoral associates needed financial support to gain research experience with a variety of metal catalysis.











