Reports: DNI352325-DNI3: Cation-Crown Ether Interactions to Control Ligand Hemilability and Catalyst Function
Alexander J. M. Miller, University of North Carolina (Chapel Hill)
Iridium complexes of the pincer-crown-ether ligand exhibit a wide range of coordination modes. The ligand can support tridentate, tetradentate, or pentatedentate binding. Experimental and computational studies were carried out to investigate the Ir-O bond strengths and to determine which ligands can displace the crown ether donors. Weak ligands such as THF do not readily displace the ether donors in pentadentate complex [(κ5-15c5NCOPiPr)Ir(H)]+ (1), but treatment with excess CH3CN forms the bis-nitrile complex [(κ5-15c5NCOPiPr)Ir(H)(NCCH3)2]+ (2). Exposing the nitrile complex to vacuum leads to reversion to pentadentate-bound 1. In the presence of sodium and lithium salts, on the other hand, the pincer-crown-ether donors are much more easily displaced. Complete conversion to the bis-nitrile complex was observed, and exposure to vacuum did not lead to any reversion to 1. Alkali metal cations clearly have a strong effect on ligand binding dynamics.
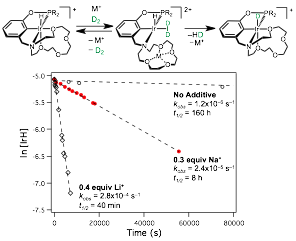
To test the ability of cations to modulate the reactivity of the iridium complex, a hydrogen cleavage reaction was carried out. Treatment of 1 with D2 gas very slowly produced the deuteride [(κ5-15c5NCOPiPr)Ir(D)]+ (1-D), with a half-life of 160 hours. Addition of catalytic amounts Na+ (0.3 equivalents) dramatically accelerated the reaction (half-life of 8 hours). Addition of Li+ gave even further rate enhancement, with a half-life of only 40 minutes. This demonstrates that the hydrogen cleavage reactivity can be tuned based on the identity of the cation (Li+ vs. Na+). Furthermore, the reaction can also be modulated based on the amount of cation present, as addition of more Na+ led to faster D2 cleavage. Monitoring the reaction showed only the starting material 1 and the product 1-D. This indicates that the process involves hemilabile ligand dissociation, while maintaining the stabilized pentadentate form of the complex. To access complexes with potential for carbonylation chemistry, we have synthesized the Ir(I) complex (κ3-15c5NCOPiPr)Ir(CO) and the Ir(III) complexes (κ3-15c5NCOPiPr)Ir(H)(CO)(Cl) and [(κ4-15c5NCOPiPr)Ir(H)(CO)]+. These species are currently being investigated for C–H activation and C–C bond formation reactivity. This research has attracted the interest of an industrial sponsor.
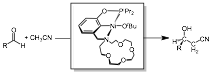
Nickel complexes of the pincer-crown-ether ligand have also been synthesized in year 2. These were readily accessed from NiBr2 to afford (κ3-15c5NCOPiPr)Ni(Br). Treatment with tert-butoxide provided (κ3-15c5NCOPiPr)Ni(OtBu), which proved to be an active catalyst for the hydrocyanomethylation of aldehydes. This C-H functionalization reaction converts readily available petroleum-derived precursors to a relatively complex hydroxynitrile product that is poised for further functionalization, demonstrating that the pincer-crown-ether ligand can support catalytic C-H functionalization reactions. Current work is focused on understanding the thermodynamics and kinetics of cation-crown interactions in Ni complexes.
Pincer-crown-ether ligands offer flexible coordination chemistry spanning tridentate, tetradentate, and pentadentate binding modes. Interconversion between these binding modes is facilitated by cation-crown interactions, which has led to the demonstration of cation-tunable hydrogen cleavage reactivity. The same ligand supports nickel-catalyzed C-H functionalization reactivity. The ACS PRF DNI award has had a major impact on the supported personnel. One supported graduate student was recognized with a departmental award and fellowship for his outstanding research efforts, which allowed the DNI award to support an additional graduate student. The DNI award has allowed the PI to present the research at the 247th ACS National Meeting and at the Organometallic Chemistry Gordon Research Conference in 2014, and a manuscript on the cation-tunable hydrogen activation at iridium has been accepted for publication in J. Am. Chem. Soc. A manuscript describing nickel-catalyzed reactions is in preparation. The generous support from the ACS has allowed the pincer-crown-ether ligand framework to form a strong foundation for our research.