Reports: DNI1052906-DNI10: Dynamic Synthesis and Assembly of Nanoporous Materials
Ronald A. Smaldone, PhD, University of Texas at Dallas
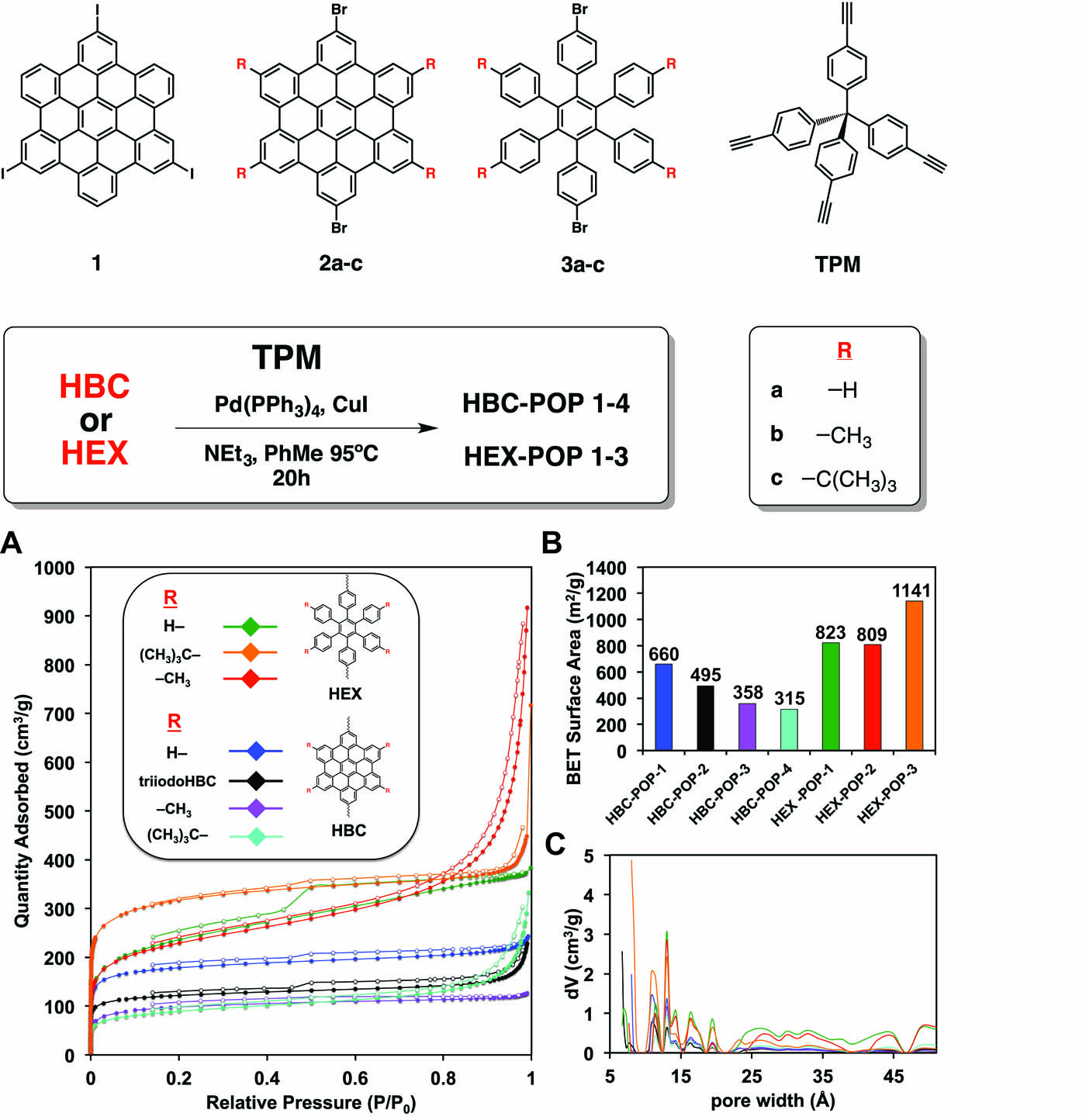
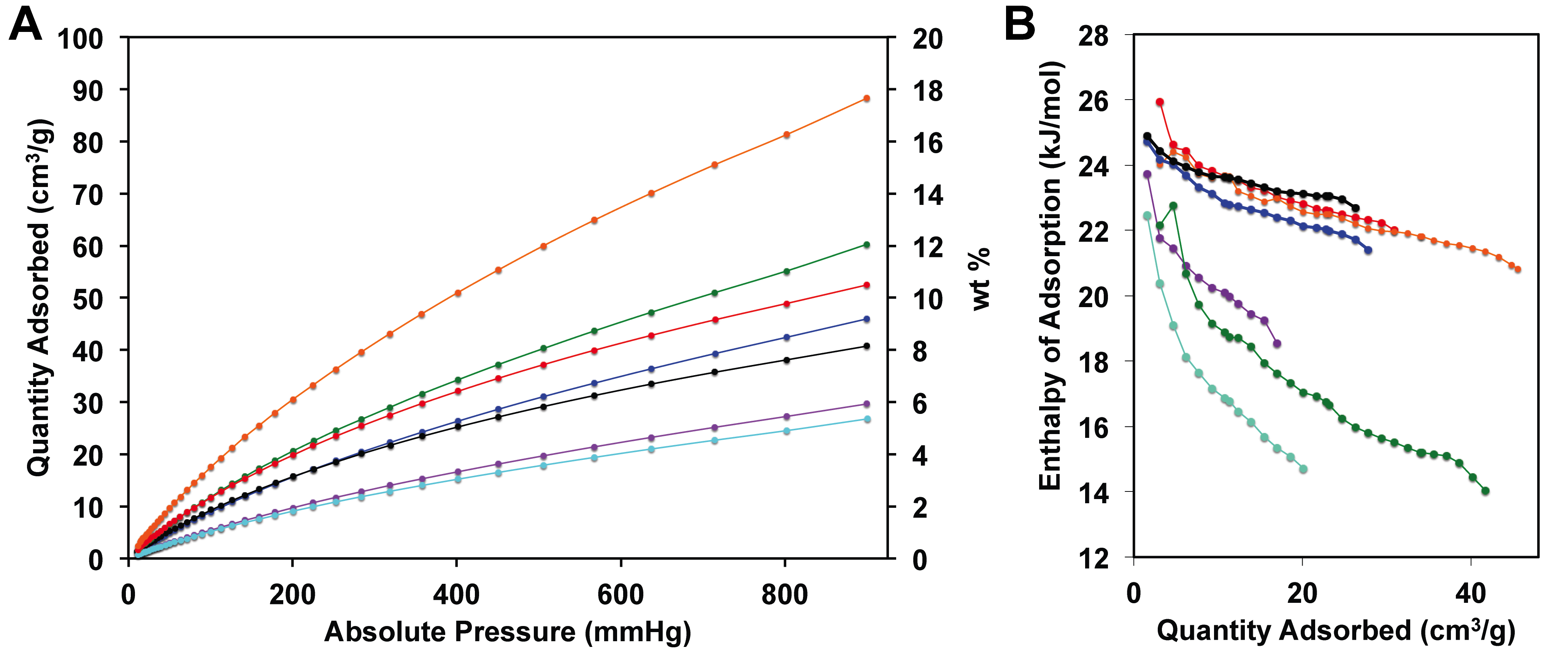
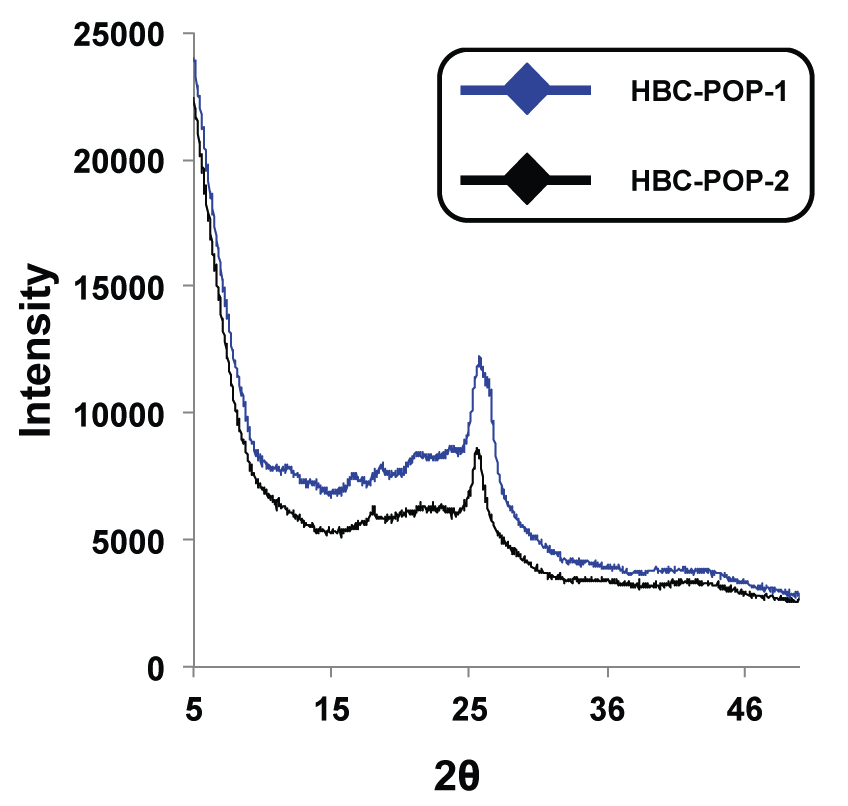
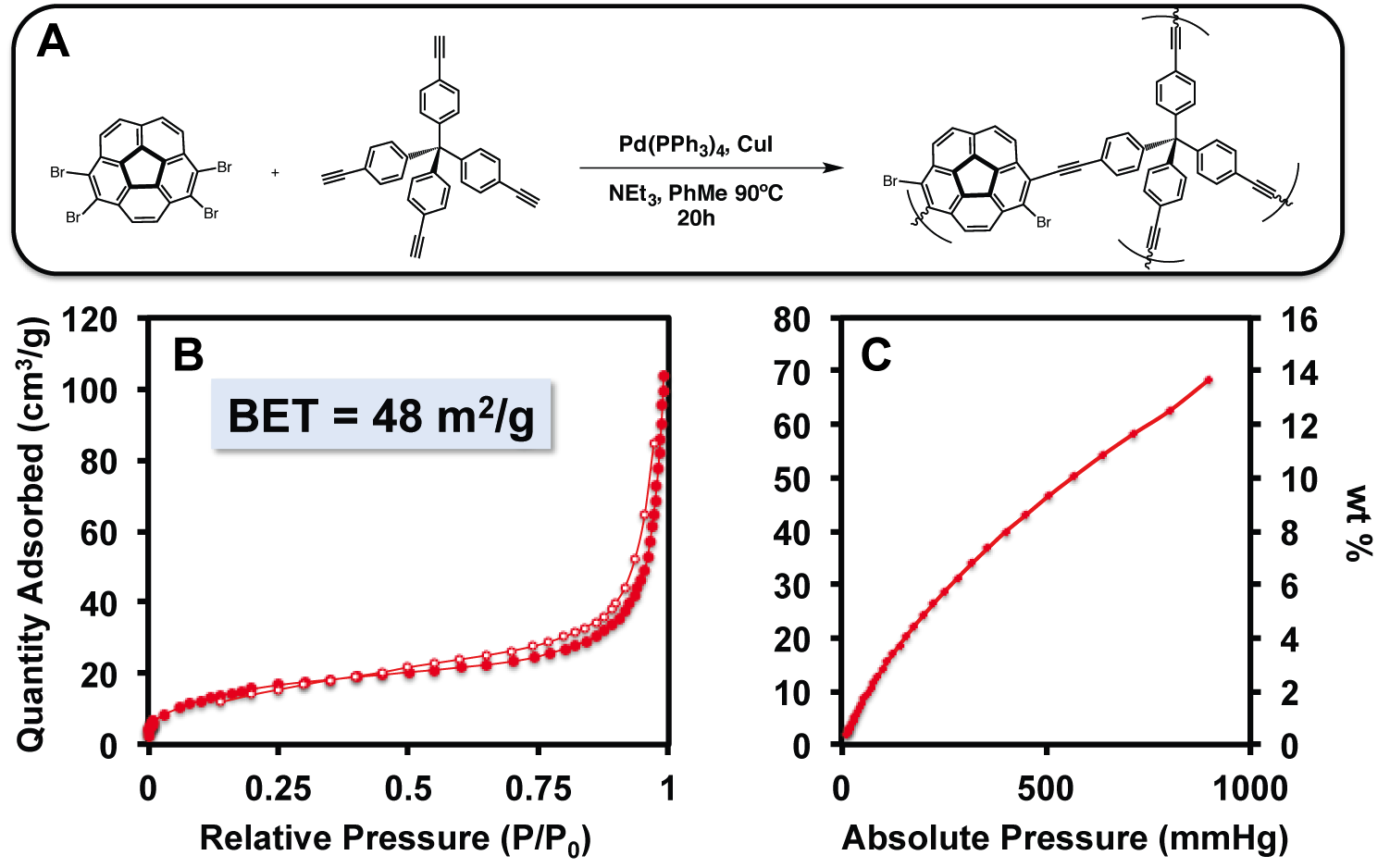
Ronald A. Smaldone, PhD, University of Texas at Dallas




Copyright © American Chemical Society