Reports: DNI653476-DNI6: Spectroscopic Investigation of Vibronic Interactions in Molecules with Low Symmetry
Jinjun Liu, PhD, University of Louisville
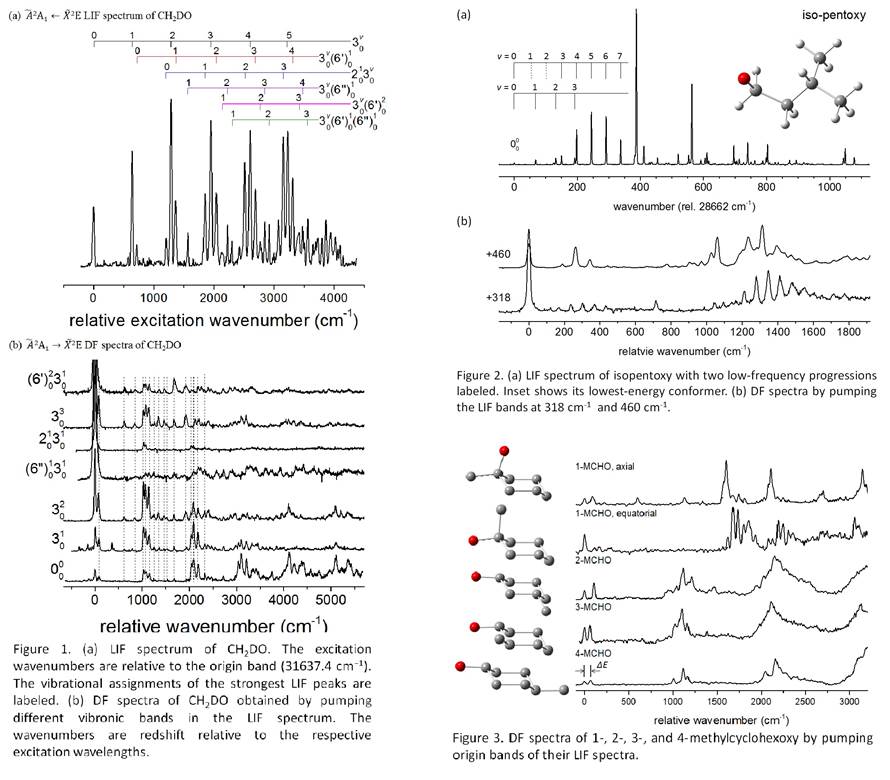
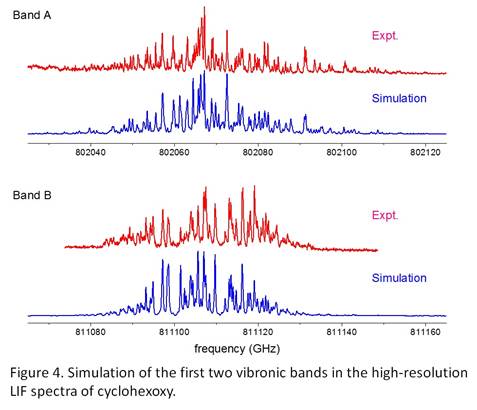
Jinjun Liu, PhD, University of Louisville


Copyright © American Chemical Society