Reports: ND753456-ND7: Supramolecular Gels and Nanocomposites with Multiple Stimuli Responsive Behavior
Christopher J. Ellison, PhD, University of Texas at Austin
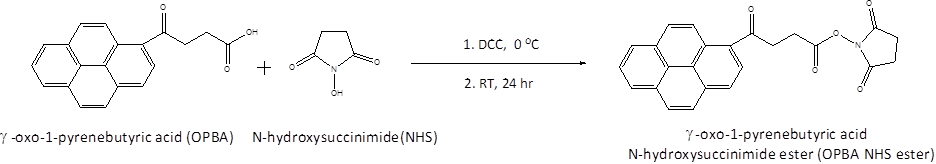
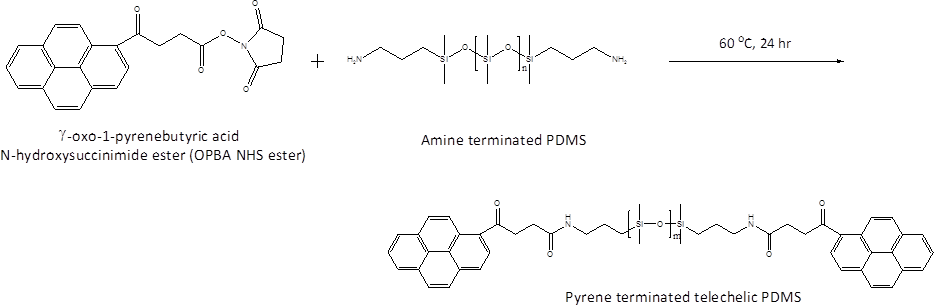

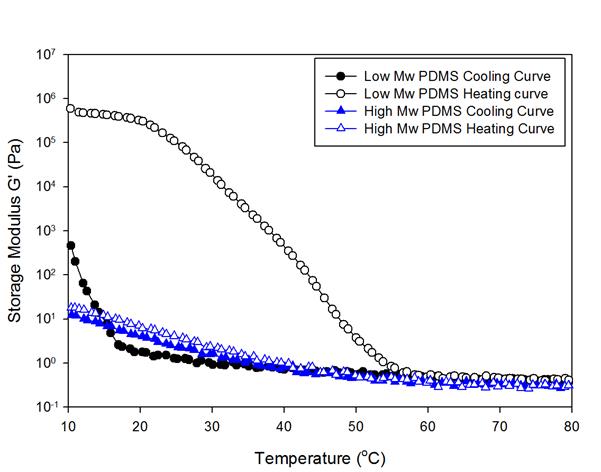
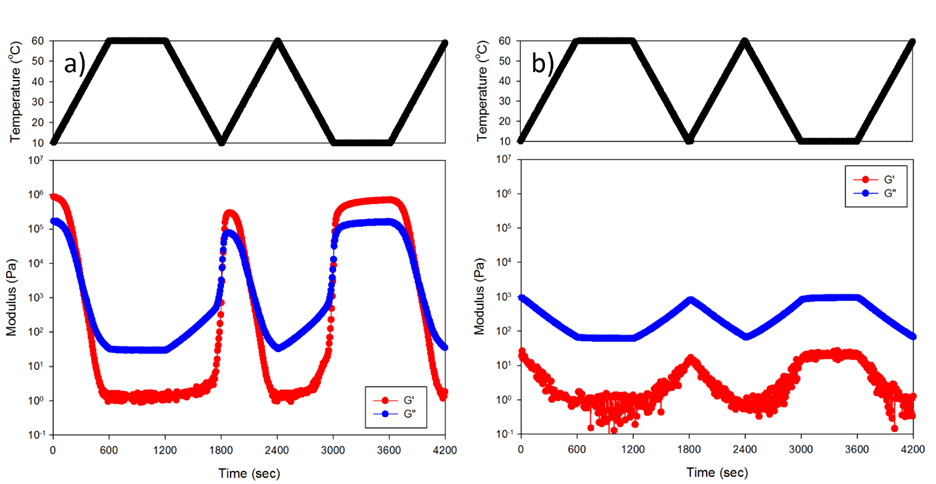
Christopher J. Ellison, PhD, University of Texas at Austin





Copyright © American Chemical Society