Reports: UR1050797-UR10: Synthesis of Novel Polycyclic Aromatic Hydrocarbons Derived from Benzotriphenylene
Kenneth E. Maly, Ph.D., Wilfrid Laurier University
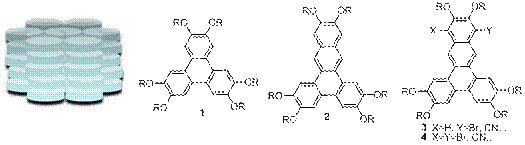
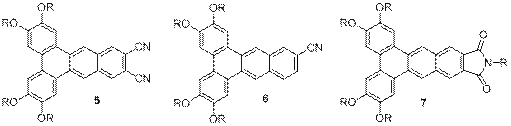
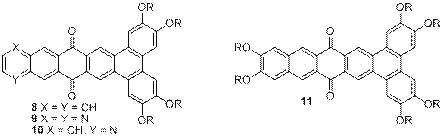
Kenneth E. Maly, Ph.D., Wilfrid Laurier University



Copyright © American Chemical Society