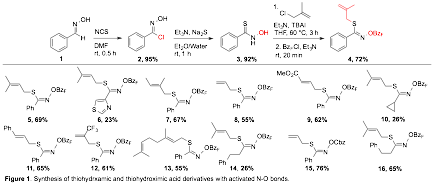
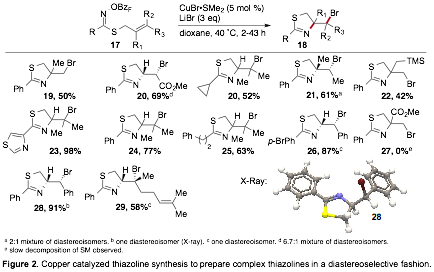
Reports: DNI153538-DNI1: Thiohydroxamic Acids as Novel Reagents for the Construction of Heterocycles
Joshua G. Pierce, PhD, North Carolina State University
This grant period has allowed
for the further development of a method to prepare thiohydroxamic
and thiohydroximic acids and utilize them in several
new bond forming reactions.
During the initial funding
period of this grant we have aggressively pursued approaches to enable the
rapid and scalable preparation of valuable heterocycles. With these thiohydroxamic acids in hand we have explored a variety of
transformations to harness their potential as synthetic reagents. In the next funding year we
plan to leverage our successful reactions to develop approaches to other
heterocyclic scaffolds and work to develop asymmetric versions of our already reported
processes.