Reports: ND1052761-ND10: Mixed Transition-Metal Sulfides and Sulfo-Fluorides for Rechargeable Li-ion Batteries
Pierre F. Poudeu, PhD, University of Michigan
During the past 18 months of the project, we have explored various materials systems. We began with the optimization of the old battery material LiTiS2, through partial substitution of Ti by Mn atoms LiTi1-xMnxS2. Although the synthesis of various LiTi1-xMnxS2 phases was successful, gain in structural stability upon charge/discharge came with a heavy cost in the capacity.
We therefore shift our attention to the Cu4-xLixS2 solid solution series as potential cathode materials for low voltage devices. This progress report focuses on the synthesis, structural characterization and evaluation of the thermal conductivity and electrochemical behavior of Cu4-xLixS2.
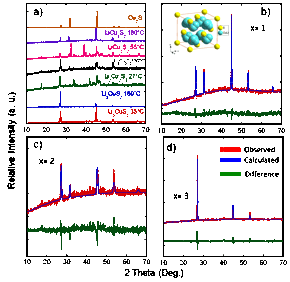
Figure 1: a) Temperature dependent X-ray powder diffraction patterns of the Cu4-xLixS2 series compared with calculated powder pattern of Cu2S. b) – d): Le Bail fitting of the high temperature X-ray powder patterns of LiCu3S2 (b), Li2Cu2S2 (c), and Li3CuS2 (d). Inset: model structure of the Cu4-xLixS2 series at high temperature.
CuS owing to its high theoretical capacity of 560 mAhg-1 and high electronic conductivity of 10-3 S/cm is an attractive cathode material for high-power rechargeable battery systems.1 Several investigations of CuS as cathode material in an Li/CuS coin cell revealed a large capacity of 530 mAhg-1 during the first discharge followed by a rapid drop in the capacity in subsequent charge – discharge cycles. 1-3 However, the cathodic reaction mechanism during the charge - discharge process is still not clear. It was proposed that the first discharge step consisted of the insertion of Li ions into the CuS crystal to form CuLixS (x < 2) followed by decomposition of CuLixS (x < 1) intermediate into CuS, Cu2S and Li2S.4
In this work, we have investigated the electrochemical behavior of the discharge materials, Cu4-xLixS2 as a cathode material in a Cu4-xLixS2/Li coin cell in order to assess the effect of Cu/Li ratio on the stability of CuLixS intermediates formed in a Li/CuS cell during the first discharge. Several samples of the Cu4-xLixS2 (x = 1, 2, 3) solid-solutions series were synthesized by reacting the elements using (1) conventional solid-state reaction in a tube furnace, (2) mechanical alloying, and (3) induction melting. X-ray powder diffraction (XRD) of the resulting products revealed a large degree of similarity between the XRD patterns of samples with a given nominal composition regardless of the synthesis method. This suggests that all three synthetic approaches are suitable to produce Cu4-xLixS2 solid-solutions. Difference scanning calorimetry (DSC) measurements showed a sharp endothermic peak at 140 °C for the sample with x = 1 and 2, which is ascribed to a structural transition. XRD measurements on various Cu4-xLixS2 samples at temperatures below and above 140 °C indicated a structural transition from the low symmetry room temperature structure to the cubic high temperature structure. The fitting of the high temperature XRD patterns of Cu4-xLixS2 using LeBail fitting methods indicates that the compounds are isostructural with Cu2S (Figure 1).
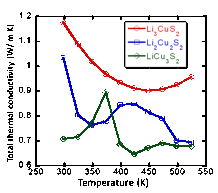
Figure 2: Temperature dependence of the thermal conductivity of Cu4-xLixS2.
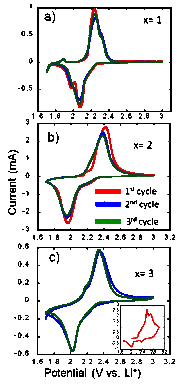
The thermal conductivity of Cu4-xLixS2 samples decreases with increasing temperature (Figure 2). The anomalous change in the thermal conductivity observed between 300 K and 410 K for the Cu-rich samples (x = 1 and 2) is associated to the structural phase transition. At 300 K the thermal conductivity of Cu4-xLixS2 samples increase with Li content from 0.7 W/m K (x = 1) to 1.2 W/m K (x = 3). Cyclic voltammetry data showed that all Cu4-xLixS2 samples are electrochemically active (Figure 3). The stability of the compounds upon charge and discharge is strongly related to the Li/Cu ratio. For example, a very large irreversible initial charge capacity of 393 mA h g-1 and a discharge capacity of 91 mAhg-1 were observed for the sample with x = 3. Interestingly, decreasing the Li content to x = 2, and x = 1 resulted in a more reversible initial capacity. The observed charge/discharge capacities are 180 mAhg-1/ 165 mA h g-1 for x = 2 and 46 mAhg-1/ 46 mAhg-1 for x = 1. These results indicate that the maximum capacity and reversibility of the charge/discharge process is achieved when the Cu:Li ratio in Cu4-xLixS2 is equal to unity (x = 2). This experimentally observed optimum composition for a stable and reversible capacity during the charge/discharge of a Li/Cu4-xLixS2 cell can be fully rationalized by taking into account the following possible cathodic chemical reactions.
Cu4-xLixS2 ^ yCuS + (4-x-y)/2 Cu2S + (x-y)/2 Li2S + yLi+ + ye- (5)
Maximum reversibility is obtained for x = 2
Figure 3: Electrochemical performance of Cu4-xLixS2/Li half-cells.
References
(1) Okamoto, K.; Kawai, S. Jpn J Appl Phys 1973, 12, 1130.
(2) Chung, J. S.; Sohn, H. J. J Power Sources 2002, 108, 226.
(3) Etienne, A. J Electrochem Soc 1970, 117, 870.
(4) Fournie, R.; Messina, R.; Perichon, J. J Appl Electrochem 1979, 9, 329.