Reports: UNI452165-UNI4: Proton-Coupled Electron Transfer in Ground-State Charge Transfer Reactions: Bi-molecular PCET and Intervalent Charge Transfer
Elizabeth R. Young, PhD, Amherst College
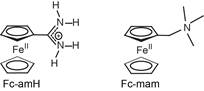
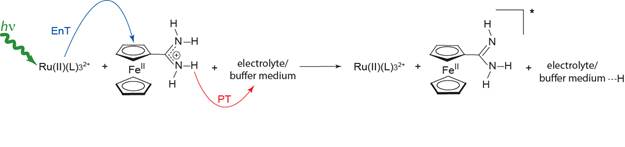
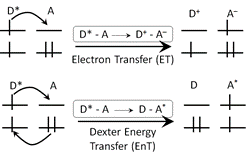
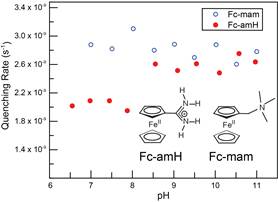
Elizabeth R. Young, PhD, Amherst College




Copyright © American Chemical Society