Reports: ND1051766-ND10: Synthesis and Characterization of Carbon Nanotube-Silicon Core-Shell Nanowire Array as Anode Material for Lithium-Ion Battery Application
Wenzhi Li, Florida International University
I. Lithiation Mechanism of Co9S8/Co–filled CNTs
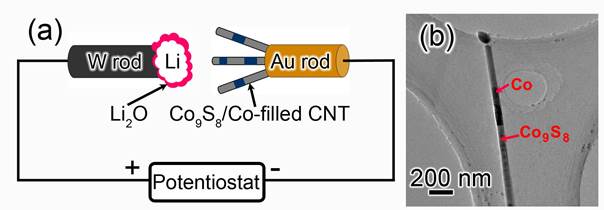
The in situ nanoscale electrochemical setup was schematically illustrated in Figure 1a. Briefly, the nano–LIB constructed in the TEM consists of three parts: Co9S8/Co–filled CNT electrode, Li counter electrode, and Li2O solid electrolyte. Figure 1b shows a TEM image of a CNT filled with crystalline Co9S8 and Co segments.
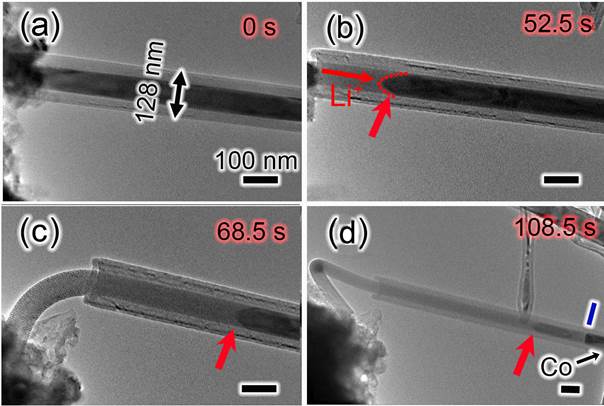
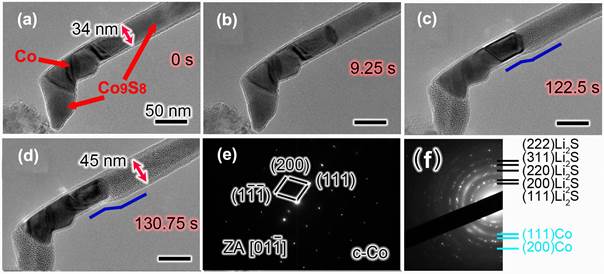
High resolution TEM image and EDS show that the electrochemical reaction of Co9S8 in LIBs can be expressed as Co9S8 + 16 Li+ + 16 e- --> 9 Co + 8 Li2S. Our research results have revealed the significant effect of the carbon cap on the lithium–storage behaviors of the filled CNT. The Co9S8/Co–filled CNT with an open end shows a notable axial elongation of the lithiated filler by 94.2% (see Figure 2) while the filled CNT with closed end shows a major radial expansion of 32.4% (see Figure 3). Intermittent swelling has been observed in the closed CNT due to the presence of Co segment, which is stable in the electrochemical cycles. During the lithiation process, single–crystalline Co9S8 nanowire converts to multi–crystalline nanowire consisting of Co nanograins and Li2S matrix, and the reversible phase conversion between Co9S8 and nanosized Co is revealed during the multiple cycles. The results revealed that the filled CNT with closed ends is favorable for the electrode material to maintain their original shape during lithiation–delithiation cycles, maintain good contact between the filling and the CNT shell, and thus benefit the electrochemical lithium–storage performance. The filler inside a CNT with open end will be extruded out of the CNT due to the large volume expansion after lithiation. Fortunately, the extruded filler will be confined by a graphite shell pulled out from the CNT by the extruded filler, and the graphite shell ensures the stability of the extruded filler during the next lithiation–delithiation cycles.
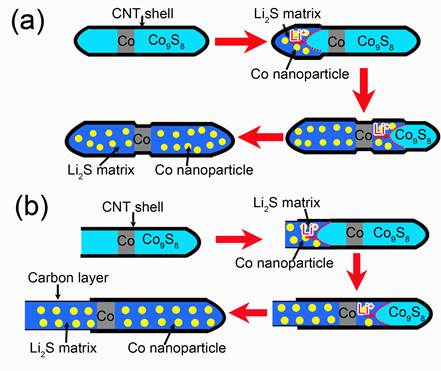
Based on the experimental results, the different lithiation behaviors of the Co9S8/Co–filled CNT with closed ends and an open end are proposed and presented in Figure 4. As for the filled CNT with carbon cap, the corresponding lithiation process is shown in Figure 4a. The pristine Co9S8/Co-filled CNT is initially straight and uniform in diameter. As the reaction front (marked by the red dashed lines) propagates from the left to the right, the CNT swells gradually. Due to the presence of short Co segment, the CNT shows intermittent expansion. Because of the suppression of the carbon cap, a small axial elongation and a significant radial expansion are presented. After full lithiation, a single-crystalline Co9S8 nanowire transforms to a multi–crystalline nanowire consisting of numerous Co nanograins embedded within Li2S matrix. Figure 4b illustrates the microstructural evolution during the first lithiation of a Co9S8/Co-filled CNT with an open end. Different from the closed CNT, as the reaction front propagates, the lithiated filler elongates and is extruded out of the CNT while still kept the similar diameter. Therefore, weakly radial expansion is demonstrated while axial elongation is notable for the lithiation of filled CNT with an open end. Co segment can move with the extending of the lithiated filler. In particular, the extended filler was still encapsulated with a graphite shell pulled out from the original CNT due to the weak intertube van der Waals force within the CNT.
Figure 1. (a) Schematic illustration of the experimental setup for in situ TEM analysis of nano–LIB. (b) TEM image of a pristine Co9S8/Co–filled CNT consisting of two different regions, Co9S8 and Co.
Figure 2. Time–resolved TEM images show the electrochemical lithiation process of an individual Co9S8/Co–filled CNT with an open end. The lithiation leads to the gradual extrusion of the lithiated filler out of the CNT due to volume expansion. The red dashed curve and arrows demonstrate the reaction front. All scale bars are 100 nm.
Figure 3. (a-d) Time–resolved TEM images show the electrochemical lithiation process of a Co9S8/Co–filled CNT with a carbon cap on the end. (e) EDS of the pure Co filler after lithiation showing no phase transformation. (f) EDS of the lithiated Co9S8 nanowire showing the obvious phase conversion to Co and Li2S. The blue lines in (c and d) denote the change of CNT diameter. All scale bars are 50 nm.
Figure 4. Schematic shows the electrochemical lithiation of a Co9S8/Co–filled CNT with closed end (a) and open end (b).
II. Sodiation Mechanism of Co9S8/Co–filled CNTs A nano sodium ion battery (nano-SIB) similar to nano-LIB was also created for the observation of the electrochemical sodiation process. The filled CNTs behave differently depending on their structures and the magnitude of the sodiation voltages. For a Co9S8-filled CNT with an open end, the sodiated Co9S8 filler shows a substantial axial elongation of 120.8% and a small radial swelling due to the extrusion of CNT walls. Contrarily, the closed CNT shows a major radial expansion of 40.6% and a small axial elongation because of the mechanical confinement of the carbon shells. After sodiation, the spacing between the carbon shells increases from 3.4 to 3.8 due to the Na+ ion insertion and the single crystalline Co9S8 filler converts to numerous Co nanograins dispersed in Na2S matrix. Compared with the gentle microstructure evolution of the CNT under small charging voltage, a strong electrochemical reaction accompanying drastic swelling and fracturing of CNT shells is observed for the CNT electrode under large charging voltage.