Reports: DNI452125-DNI4: Watching Single Catalyst Molecules in Action
Randall H. Goldsmith, PhD, University of Wisconsin (Madison)
The supported work focuses on using single-molecule
fluorescence microscopy to elucidate fundamental mechanistic processes in
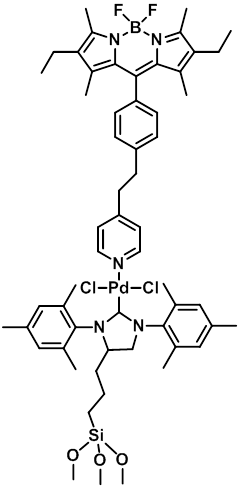
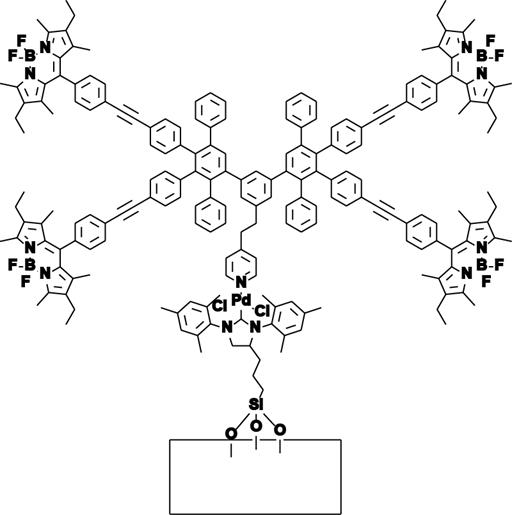
individual, working catalyst molecules. We have focused on palladium catalysts due to their
synthetic importance and wide applicability, and N-heterocyclic carbene (NHC)
complexes in particular due to their high activity and chemically stable metal-heteroatom
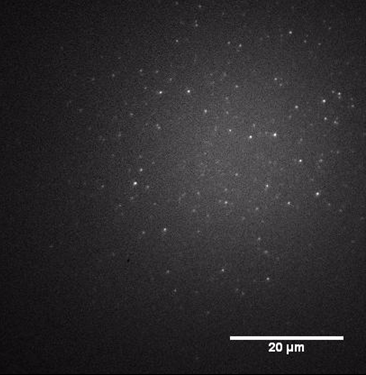
bond which can be used as a robust linker to a surface anchor. Armed with the catalysts described above, we used our new
imaging setup to observe individual catalyst molecules, beginning with the
mono-labeled complex, producing images like that shown in FIGURE 3.