Reports: DNI1053638-DNI10: Bi-Compartmental Targeting Vehicles for In-Situ Catalytic Recovery of Hydrocarbon Molecules
Ning Wu, Ph. D, Colorado School of Mines
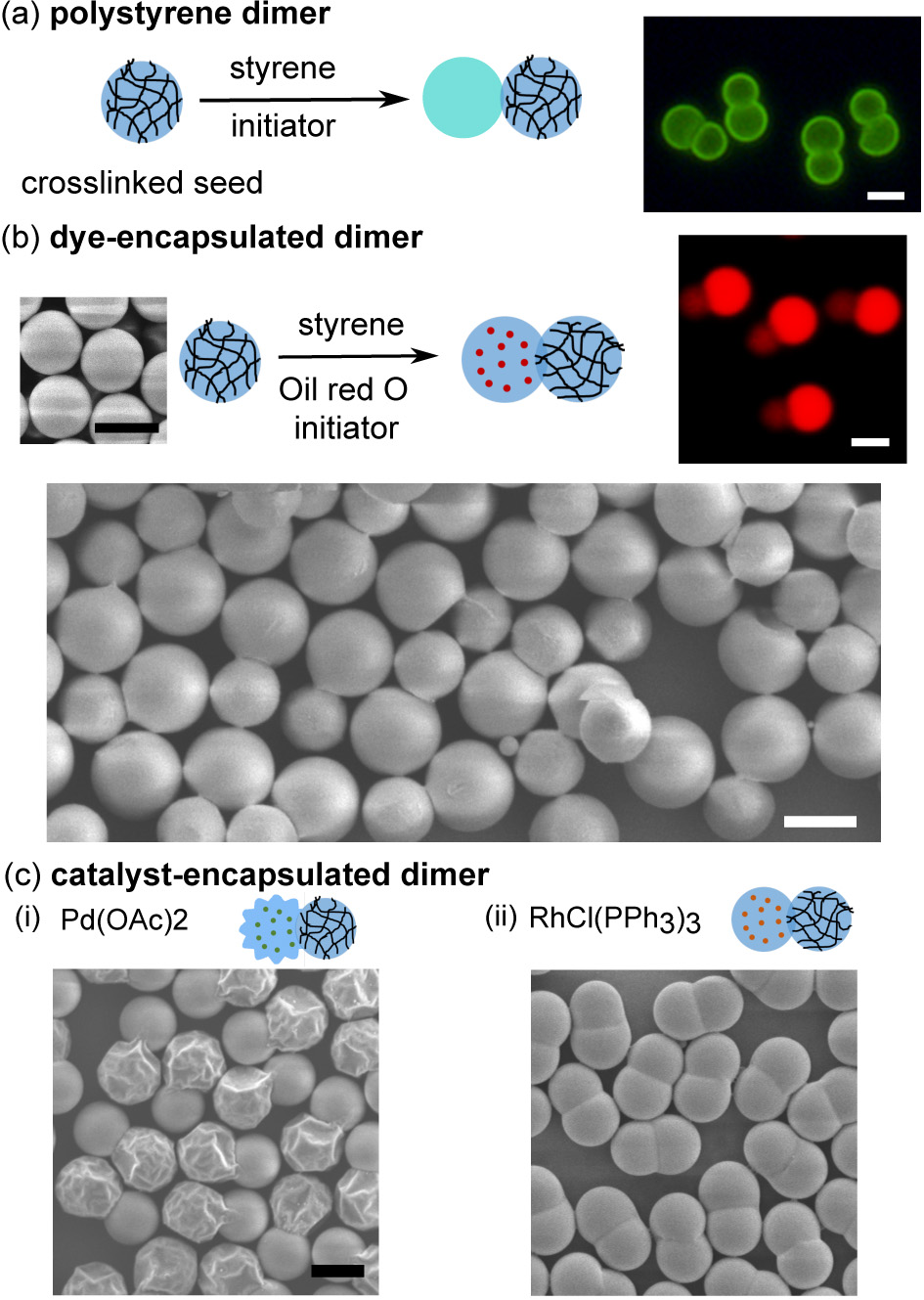
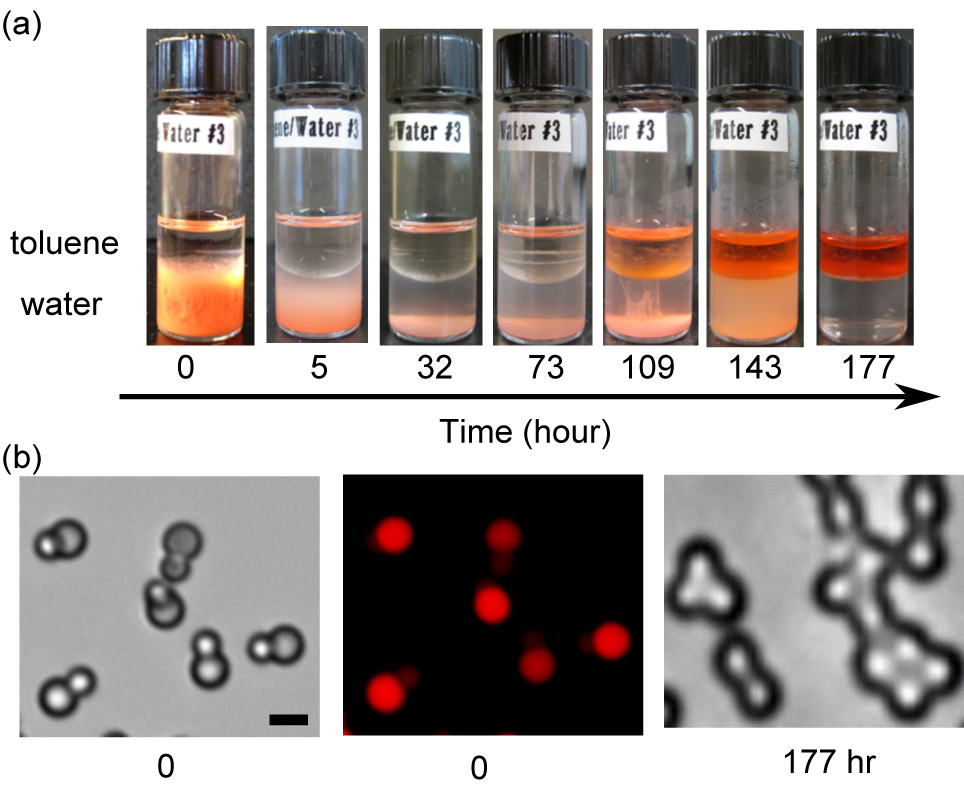
Ning Wu, Ph. D, Colorado School of Mines


Copyright © American Chemical Society