Reports: UR452099-UR4: Mechanisms and Dynamics of Carbene Additions to Anti-Bredt Olefins
Dina C. Merrer, PhD, Barnard College
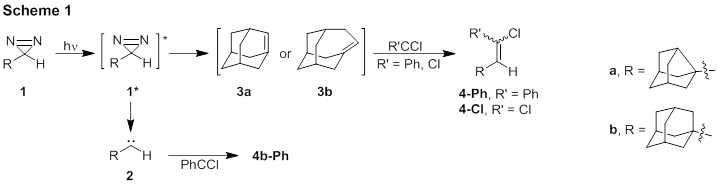
The co-photolysis in pentane of diazirines 1 each with phenylchlorodiazirine (6) and 1a with phenanthride 5-Cl yielded 4a-Ph, 4a-Cl, and 4b-Ph. These products were characterized via NMR (1H, 13C, DEPT-135, HSQC, HMBC, and NOESY) and HRMS. The yield of 4a-Ph is 11%, of 4b-Ph is 6%.
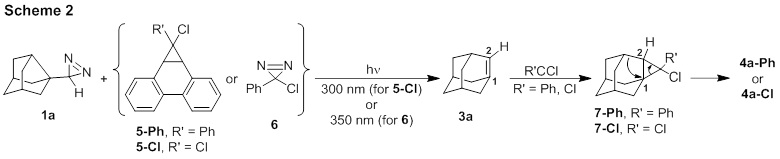
We propose the
formation of 4a via the mechanism in Scheme 2. To investigate this
path, we previously monitored the photolysis of 1a + 6 via 1H
NMR spectroscopy. We did not expect to see 3a by NMR due to its
microsecond lifetime at room temperature,
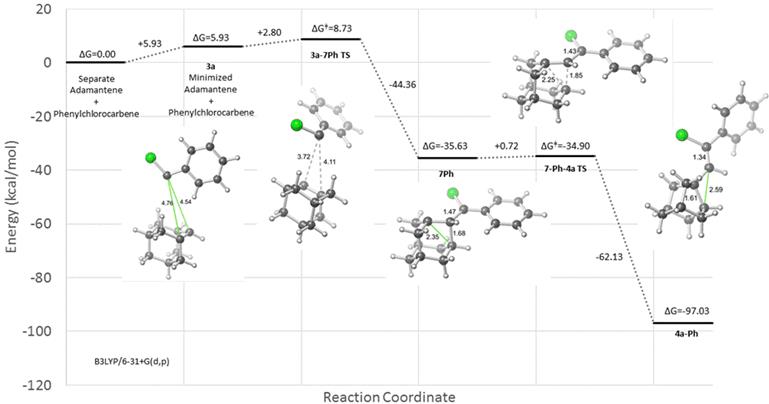
This report
covers the period 9/1/13-8/31/14. During this time, three Barnard College
undergraduates worked on this project, none of whom were funded directly by the
PRF. The reasons why no students were funded by PRF were because two students
were funded by individual summer research fellowships which was an honor (HHMI
to E. Dalchand, ConEdison to K. Francisco), and the third student (A. Scorese) was
funded by the PI’s single-investigator NSF grant nearing expiration. All three
of these students and an additional one will continue in the PI’s lab as
seniors, a junior, and a sophomore. E. Dalchand, S. Tsuno, and C. Buzard
presented a poster on this work at the March 2014 ACS National Meeting in Dallas,
TX. The PI presented a poster on this work at the June 2014 Reaction
Mechanisms Conference in Davis, CA. The very promising recent computational
results have rightly delayed submission of manuscripts on chlorocarbene additions to each
of adamantene and adamantylcarbene. We project these manuscripts to be
submitted by January 2015. The co-authors on these two manuscripts will include
11 Barnard College undergraduates. The projected publications will assist
substantially in the PI’s efforts towards promotion from associate to full
professor.











