Reports: DNI551723-DNI5: Mechanism of Oxidative Dehydrogenation of Alkane on Supported Vanadium Oxide
Zhenrong Zhang, PhD, Baylor University
Narrative Progress
Report
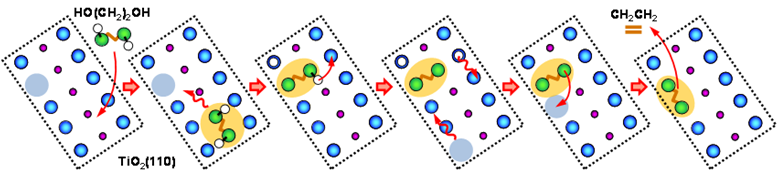

Zhenrong Zhang, PhD, Baylor University
Narrative Progress
Report


Copyright © American Chemical Society