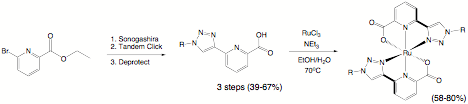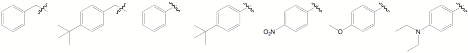Reports: UR352779-UR3: Triazole-Containing Tridentate Anionic Chelators for Lanthanide and Group VIII Organometallic Complexes
James T. Fletcher, PhD, Creighton University
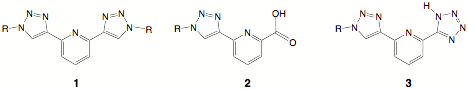
Figure 1. Figure 2. This chelator was
successfully shown to chelate Ru(II) in a 2:1 manner following a traditional
approach used to prepare 2:1 coordination compounds with neutral tridentate
chelators such as terpyridine. The tetrazole-containing
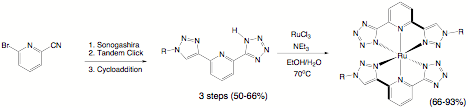
chelator 3 was prepared in a
largely analogous manner as that used to prepare 2. Figure 3. Each approach was
demonstrated feasible for a range of benzyl and aryl substituents, as
summarized in Figure 4. Figure 4. These coordination compounds
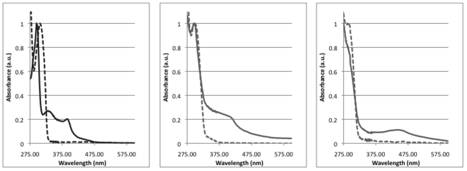
were sufficiently soluble to allow characterization by UV-Visible
spectroscopy. Figure 5. With a modular synthetic
approach to prepare analogs of chelators 2 and 3 established in year
one, work during the second year of this grant will focus on two major
areas. In year one, this project
has directly supported three different undergraduate research students who have
conducted research during both the summer months and academic semesters.