Reports: ND1053140-ND10: Nanofabrication of Porous Membranes for Separations of Gaseous Energy Carriers Under Conditions of Single-File Diffusion
Kirk J. Ziegler, University of Florida
Sergey Vasenkov, University of Florida
 |
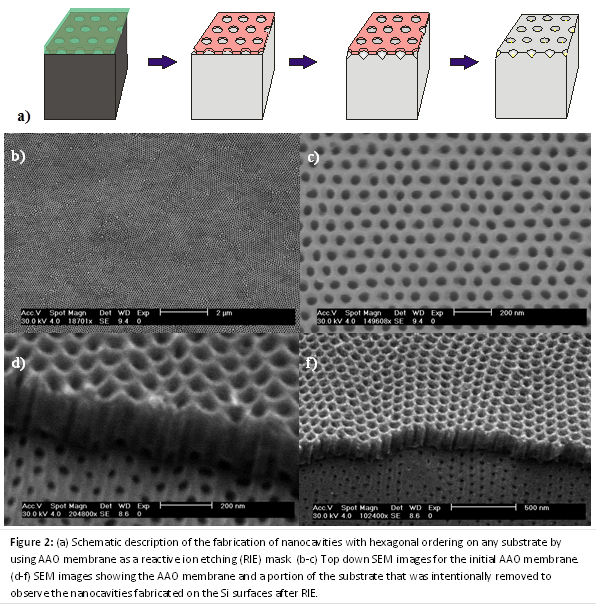
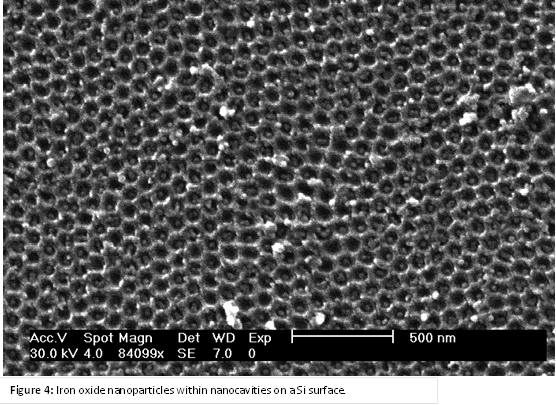
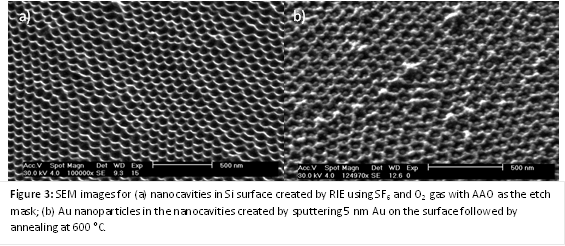
(1) Fabricate porous membranes with dimensions below 10 nm. Highly ordered porous membranes of anodic aluminum oxide (AAO) were fabricated using 2-step anodization in selenic acid. As shown in Figure 1, the mean pore diameter was measured to be 9.4 nm, which is a noticeably smaller distribution in size than previously reported values (range of 7.1 – 13.4 nm). During the upcoming year, further reductions in pore diameter will be achieved by the synthesis of carbon nanotubes within the pore channels. The pore diameter will be controlled by the number of shells of the CNT. The shell walls will also be atomically flat, which is important for observing single-file diffusion.
(2) Fabricate sacrificial nanowires for pore formation
via monodisperse catalyst particle arrays. Although current approaches achieve
monodisperse catalyst particles on a surface, the particles tend to coalesce
and undergo Ostwald ripening during the growth of nanowires. Therefore, the
dimensions of the nanowires rarely match the initial diameter of the particles.
To overcome these problems, highly ordered AAO membranes are used to
 Preliminary
measurements of single-file diffusion in nanoporous membranes. PFG-NMR at
high field and high gradients is uniquely suited for distinguishing between single-file
and normal diffusion. These studies have focused on model systems prior to
studies on the membranes fabricated above.
Preliminary
measurements of single-file diffusion in nanoporous membranes. PFG-NMR at
high field and high gradients is uniquely suited for distinguishing between single-file
and normal diffusion. These studies have focused on model systems prior to
studies on the membranes fabricated above.











