Reports: UR652486-UR6: Electron-Induced Reactions: From Methods for Characterization of Intermediates to Mechanisms
Thomas Sommerfeld, PhD, Southeastern Louisiana University
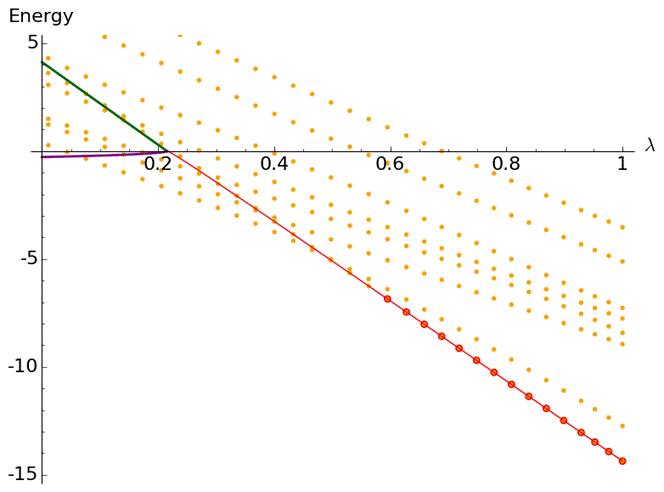
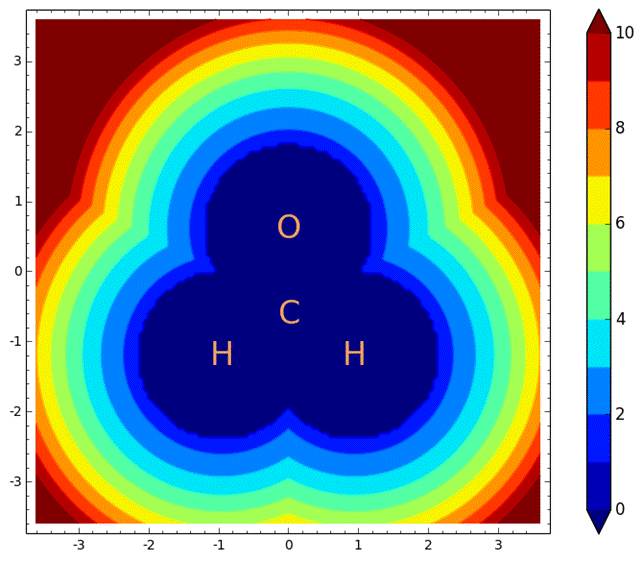
Thomas Sommerfeld, PhD, Southeastern Louisiana University


Copyright © American Chemical Society