Reports: UNI351903-UNI3: Biomimetic Catalysts for Hydrogen Production and Carbon Dioxide Activation
Adriana Dinescu, Wilkes University
This research has focused on Cu-Mo binuclear complexes and similar organometallic compounds that mimic the Cu-Mo metallocluster found in the active site of carbon monoxide dehydrogenase (CODH) from O. carboxidovorans. The role of the Mo-Cu metal cluster found in the active site of CODH is to catalyze the reversible oxidation/reduction of CO to CO2 (eq.1).
While the forward reaction is relevant for hydrogen (H2) production and similar to water gas shift (WGS) reaction, the reverse reaction could be applied to convert carbon dioxide (CO2) into useful products such as methanol and dimethyl ether. During the second year of PRF support, our research advances were (1) to continue the investigation on the structural and mechanistic characteristics of various binuclear catalysts for H2 production and CO2 activation, and (2) to assess the redox-active nature of dithiolene, diimine and oxalate ligands that could influence the reactivity of metal centers.
The work has been done by employing molecular modeling and electronic structure calculations. All calculations have been performed using density functional theory and pseudo-potential basis sets: PBE0 with 6-311++G(d,p) for H, C, N, and O; modified LANL2DZ for all transition metals; LANL2DZ augmented with diffuse functions for S.
Results
Energy profiles of the catalytic mechanism for different metal clusters were calculated using density functional theory. While the active site of CODH contains a Mo-Cu cluster, we have also tested Cr-Cu, W-Cu, Cr-Ag, Mo-Ag, W-Ag, Cr-Au, Mo-Au and W-Au metal clusters while preserving the non-innocent dithiolene ligand, which is a smaller model of the molybdopterin cofactor present in the native active site of CODH. Then, we replaced dithiolene with other non-innocent ligands, diimine and oxalate. In the first year of PRF support, we calculated eight energy profiles, while in the second year we calculated nineteen energy profiles. The first energy profile, which mimics the active site of the native CODH (Mo-Cu with a dithiolene ligand), showed the formation of a very stable intermediate. Since this thiocarbonate intermediate (-44 kcal/mol, Figure 1) is much more stable than reactants or products, it would stop the catalytic cycle of the WGS reaction. To avoid this energy trap we designed and tested several binuclear catalysts.
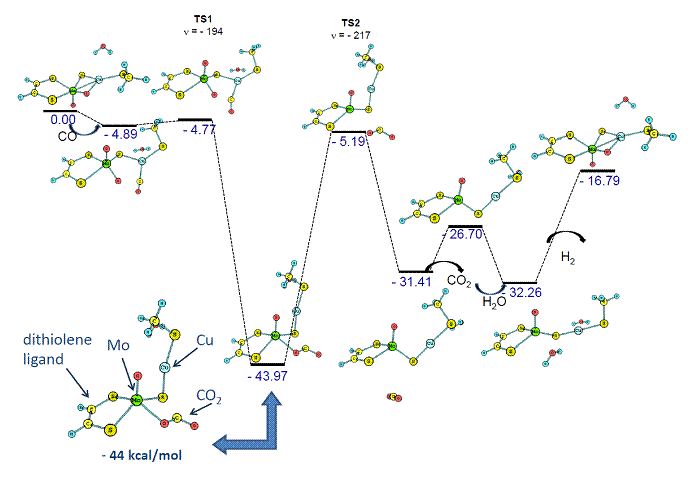
Figure 1. Energetics (enthalpy in kcal/mol) of the WGS reaction pathway using a catalyst that contains the Mo-Cu metallocluster and dithiolene ligand (low energy intermediate is shown on the left).
Most analogs of the dithiolene-Mo-Cu cluster showed a better performance. After calculating their energy profiles, we were able to find suitable catalysts that would avoid the low energy trap of the thiocarbonate intermediate (Figure 2). Several catalysts formed more reactive intermediates that effected in lower energy barriers. The most promising results were obtained with the following catalysts: dithiolene-W-Cu (-15 kcal/mol), dithiolene-Mo-Au (-18 kcal/mol), dithiolene-W-Au (-19 kcal/mol), diimine-Mo-Cu (-15 kcal/mol), diimine-Mo-Ag (-17 kcal/mol), diimine-W-Ag (-14 kcal/mol), and all six oxalate complexes (from -17 kcal/mol to -20 kcal/mol). All Cr-containing catalysts formed intermediates that were much stable (from -60 to -75 kcal/mol) than the initial one, generated with the dithiolene-M-Cu complex. For this reason, we did not include these energy results in Figure 2.
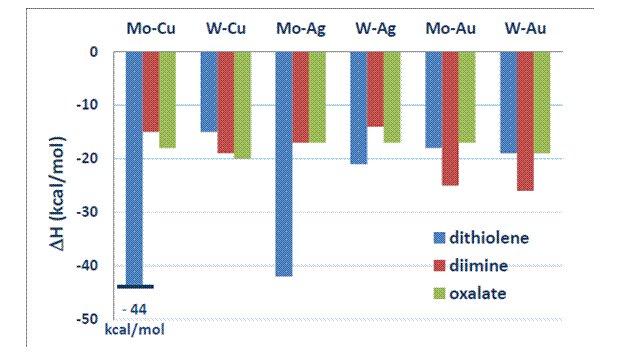
Figure 2. Energetics (enthalpy in kcal/mol) of the thiocarbonate intermediate using a variety of catalysts that are analogs of the dithiolene-Mo-Cu metallocluster.
Currently, we are working on structural and energy refinements of the selected catalysts. These refinements include intrinsic reaction coordinate (IRC) calculations that connect each transition state with the reaction intermediates. Our future work will explore a larger range of catalysts.
Student Participation and Dissemination
This PRF award was used to support the research of seven undergraduate students during the summers of 2012, 2013 and 2014. Four of these students have given poster presentations at the ACS national meetings (fall 2012, spring 2014 and fall 2014). Other two students will present their research at the 2015 annual symposium organized by King’s College, Misericordia and Wilkes Universities. We will also attend the 249th ACS National Meeting in Denver.











