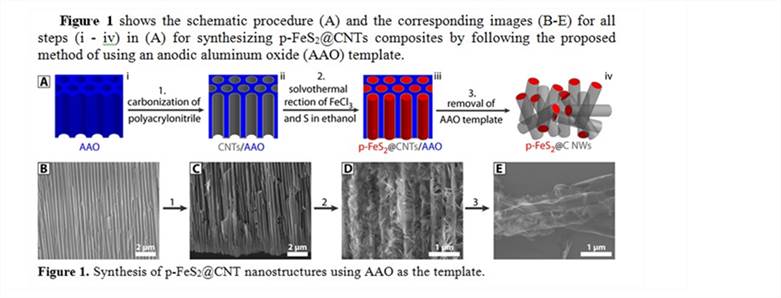
Reports: DNI1052877-DNI10: Improving the Cyclability of Iron Pyrite (p-FeS2) Cathodes in Lithium Ion Batteries Through Carbon Nanotube Encapsulation
Yongan Yang, PhD, Colorado School of Mines
The development of iron pyrite (p-FeS2) cathode for lithium ion batteries (LIBs) at room temperature is attractive. The key challenge is how to achieve high cyclability, for which the impeding factors have typically been ascribed to: 1) the poor electrical conductivity of the discharge product, Li2S; 2) irreversible chemical reactions between p-FeS2 and the electrolyte; 3) the loss of materials due to the formation of soluble polysulfides; and 4) the partial loss of the electrical contact between p-FeS2and the current collector due to the volume-fluctuation during the discharging/charging cycles.
2. Goal of the Project
Our goal in this ACS-PRF grant is to develop an efficient mitigation strategy to improve the cyclability of p-FeS2 cathodes through carbon nanotube encapsulation.
3. Research Progress
3.1 Success of synthesizing p-FeS2@CNT nanostructures through a template-induced encapsulation method
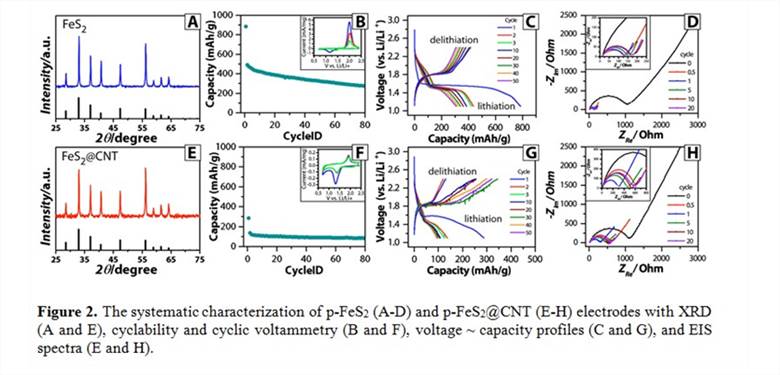
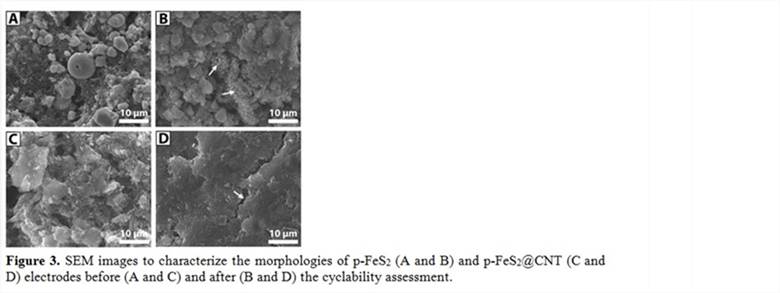
3.2 Systematic assessment of the battery performance Figure 2 shows the systematic characterization and assessment of both p-FeS2 (A-D) and p-FeS2@CNTs (E-H) electrodes. The X-ray diffraction patterns in A and E shows the characteristic features of p-FeS2. The cyclability of p-FeS2 electrode is significantly better the p-FeS2@CNT electrode (B and F). Their cyclic voltammograms (the insets in B and F) are similar; while the abnormally high reduction peaks in p-FeS2 electrodes need further study to fully understand. The voltage ~ capacity profiles (C and G) show that p-FeS2 electrodes present the characteristic lithiation/delithiation plateaus, which are not observed in p-FeS2@CNT electrodes. The electrochemical impedance spectroscopy (EIS) spectra (E and H) show that these two electrodes are comparable initially and in the subsequent cycles the charge transfer resistances (the amplitude of the semicircle in the ZRe axis) of p-FeS2@CNT electrodes are significantly larger than those of p-FeS2 electrodes. The scanning electron microscopic (SEM) images in Figure 3 show that p-FeS2 electrodes start with a mixture of large spheres (a few micrometers in diameter) and fine particles and change to flower-petal like surfaces after 20 cycles. In contrast, p-FeS2@CNT electrodes mainly show the morphology of the additive materials in the beginning and show cracks after 20 cycles.
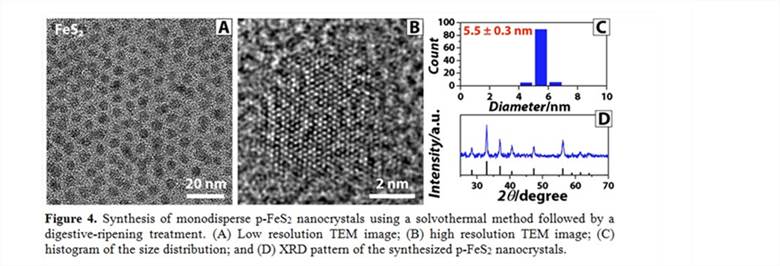
The study here shows that the p-FeS2@CNT composites unfortunately did not accommodate the volume fluctuation of p-FeS2 as expected, which could be assigned to the low mechanical stability of the CNT shells. The challenge was assigned to the incompatibility between thin CNT shells of ~3 nm in thickness and large p-FeS2 diameters of ~200 nm. Therefore, we began pursuing highly dispersed smaller p-FeS2 nanocrystals as well as developing new types of carbon encapsulation. 3.3 Advance in synthesizing monodisperse FeS2 nanocrystals Monodiperse, spherical, colloidal, and phase-pure p-FeS2 nanocrystals (5.5 ± 0.3 nm in diameter) have been synthesized via a scalable solvothermal method using iron (III) diethyldithiocarbamate as the precursor, combined with a post digestive ripening process (Figure 4). Through this study, we have verified a hypothesis that solvothermal syntheses can also produce colloidal FeS2NCs if achieving high precursor solubility, efficient mass transport, and sufficient surface stabilization.
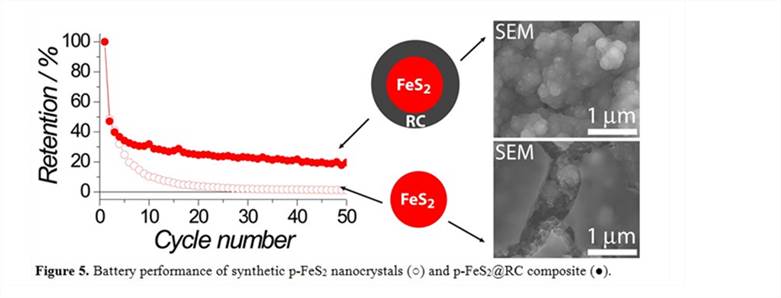
3.4 Endeavor of developing new types of carbon encapsulation The new carbon encapsulation was performed by a hydrothermal treatment of premade p-FeS2 nanocrystals with glucose as the carbon precursor to produce p-FeS2@polymer composite and then pyrolyze the polymer to obtain p-FeS2@RC composites (RC = resilient carbon). The electrode performance is indeed superior in capacity retention compared with the p-FeS2 counterpart, supported by morphological characterization (Figure 5). The remaining issues associated with the current system have also been identified and will be studied in the future.
4. Broader Impacts
This project in the past one and half years has resulted in multi-faceted impacts on all participants. We believe these impacts will continue to manifest in our future research and life.
By leading this project, the PI has improved his scholarship in many aspects, including the tactics for approaching scientific problems, the skills of mentoring graduate/undergraduate students, the capability of developing new projects, and national/international recognition. In 2013, the PI delivered four invited talks on this project at four prestigious research institutions. In 2014, he contributed an oral presentation at the 18thAnnual Green Chemistry Engineering Conference, because of which he has been invited to submit one review paper.
Three graduate students, all females, have been partially supported by this grant. The major researcher, Tara Yoder, has submitted two first-author manuscripts, both of which are in revision. Her established passion and competency in scientific research can be embodied by her prize-winning presentation at the CEER conference at Golden, CO and the waived registration fees to attend the 2014 ECS meeting in Mexico.
Two unpaid undergraduate students have also participated in this project. Mr. Tussing, who worked in 2013, has coauthored on both of the submitted manuscripts. His excellent performance was recognized by a Departmental research award. A month ago, he sent me an email to reiterate his appreciation for working with us and the positive impacts on him.
This project has also helped us to establish collaborations with two colleagues in our department and one colleague at UCLA.