Reports: DNI1052461-DNI10: The Relationship Between the Supercooled Liquid Region, Elemental Enthalpy of Hydride Formation, and Hydrogen Embrittlement in Amorphous Metallic Thin Films
Mary Laura Lind, PhD, Arizona State University
Introduction
Experimental
Results and future work
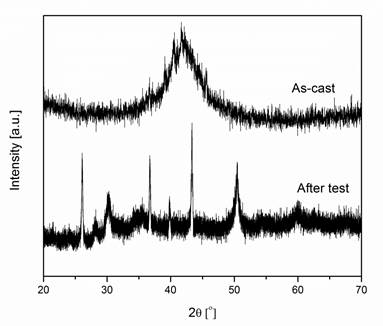
Figure 2 XRD pattern of
as-cast membranes and after hydrogen permeability test.
Impact
We weighed and arc-melted high purity raw metals to yield master ingots of the
desired amorphous compositions. Then we used splat quenching to synthesize ~ 40
micrometer thick membranes of selected compositions. Splat quenching is a
technique that rapidly cools a molten metal at rates up to 10^6 K/s. To promote
the hydrogen dissociation on the surface, we sputtered thin layers of Pd onto
both sides of the splat quenched membranes. After sputtering, we annealed the
membranes in vacuum oven at 523K to promote the adhesion of Pd to the surface.
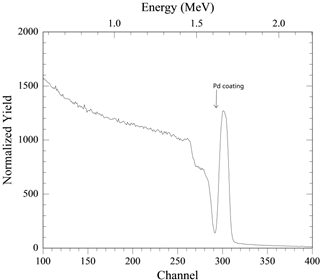
We used Rutherford backscattering spectrometry (RBS) to estimate the thickness
of the sputtered Pd layer.