Reports: UR552145-UR5: Synthesis of Ruthenium Dioxide Nanoparticles and Clusters with Tailored Size-and Surface-Dependent Properties for Enhanced Catalytic Applications
Vladimir Kitaev, Wilfrid Laurier University
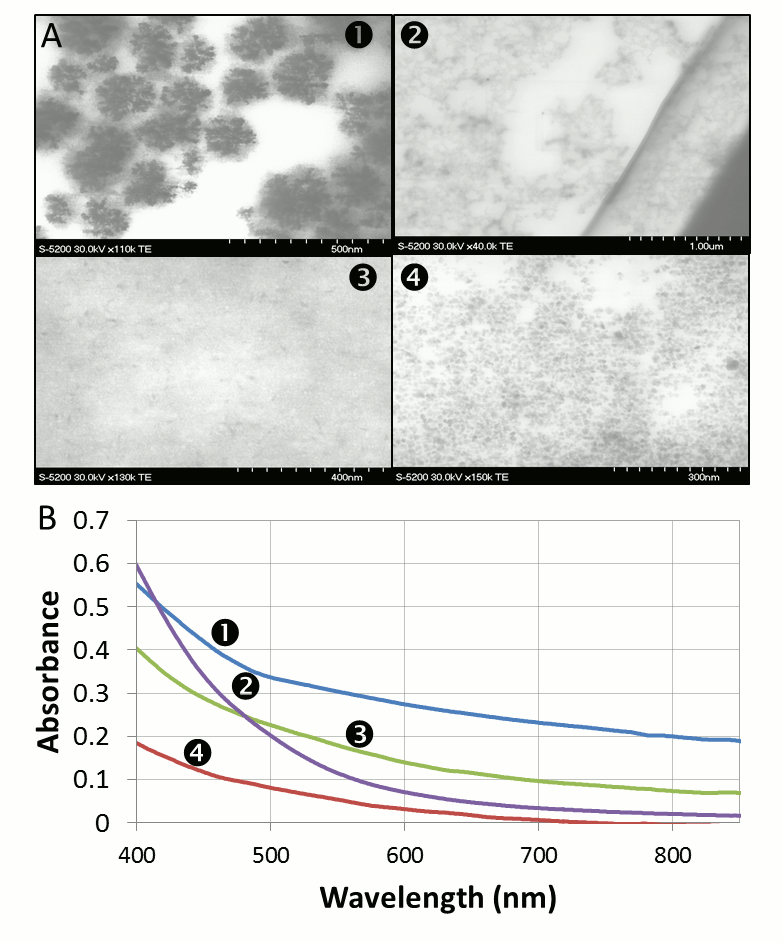
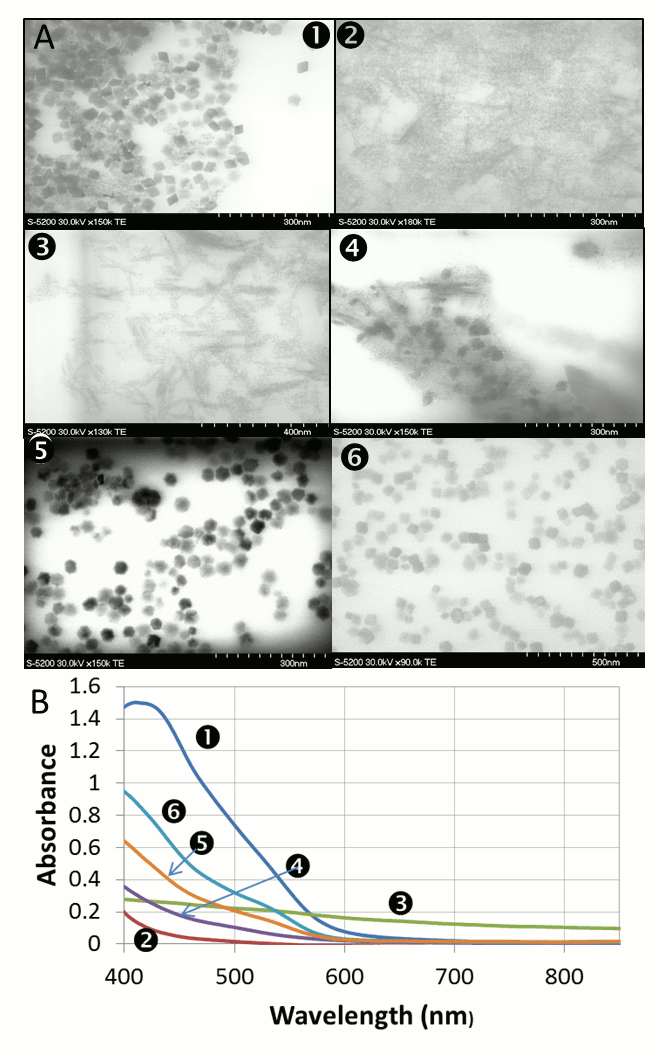
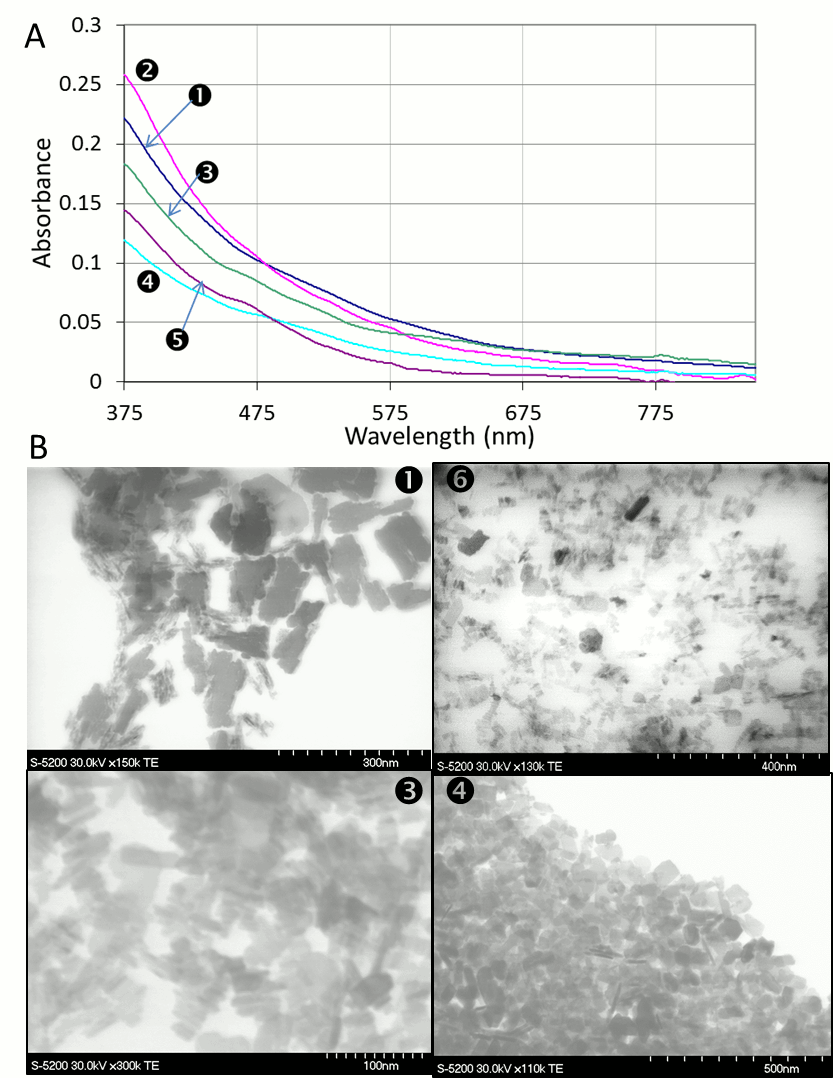
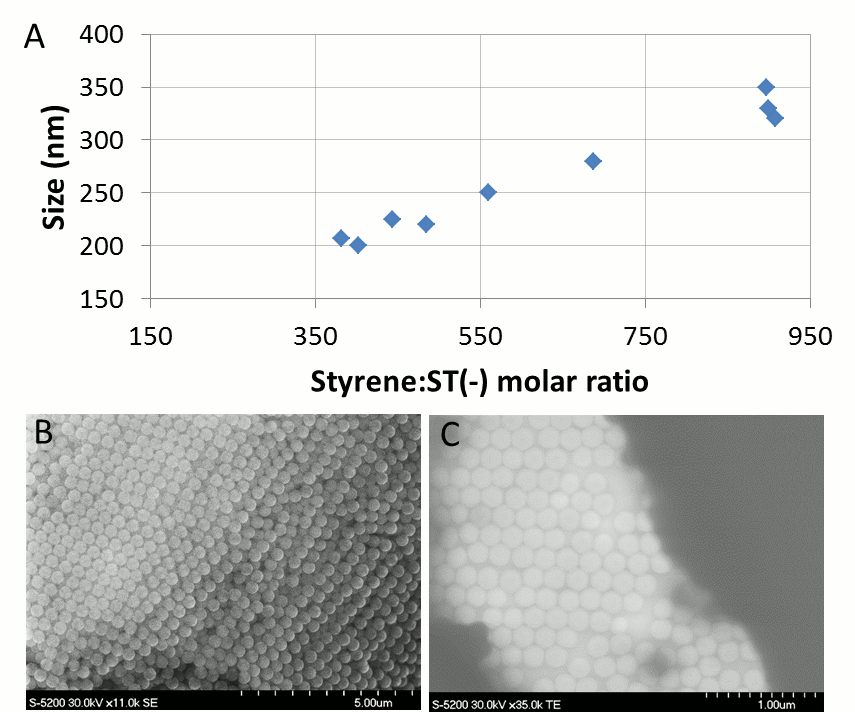
Vladimir Kitaev, Wilfrid Laurier University




Copyright © American Chemical Society