Reports: UR351085-UR3: Fundamental Chemistry of the Re(CO)3(H2O)3+ Synthon
Richard Herrick, PhD, College of the Holy Cross
For several years, we have been actively investigating the preparative chemistry of Re(CO)3+ chloride and bromide compounds. We have been exploring the biological chemistry of rhenium tricarbonyl compounds as models for technetium, and Re(CO)3+ iodides may also prove to be ideal as imaging agents due to their inert nature. Additionally, iodide compounds are also employed in many medicinal applications. Iodide-131 compounds are used to treat hyperthyroidism and some types of thyroid cancer,2 and aromatic iodinated drugs are used as contrast agents to enhance the visualization of soft tissues with X-rays.3
Since there are few examples of group 7 organometallic compounds with iodide ligands we have begun an exploration of this area.
Scheme 1. Reaction scheme and numbering system for iodide compounds.
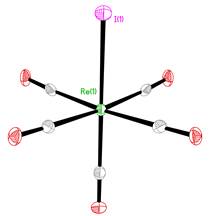
We began by examining the simple reaction chemistry of Re(CO)5I. We found an improved synthesis of Re(CO)5I from Re(CO)5(OTf) 1, OTf = triflate along with subsequent reactions forming various diimine derivatives. We also found a unexpected crystal structure obtained from crystals of Re(CO)5(OTf), a crystal structure of Re(CO)5I that differs from the previous report, Fig. 2,4 along with four other rhenium (I) carbonyl iodide crystal structures. Additionally, we prepared the pyridine 2-carboxyaldehyde derivative 7 via an alternate route through the Re(CO)3(H2O)3+ ion. Fig 1 shows the numbering scheme and reactions discussed in this report.
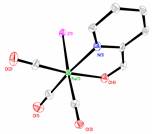
Compound 2 was prepared as the gateway compound to various new derivatives. Its synthesis works well from 1 with good yields resulting in pure product. Compound 2 reacts in ways similar to Re(CO)5Cl and Re(CO)5Br with analogous products produced. The main difference is that reactions are significantly slower. We have found that a four-hour reflux in methanol is sufficient to complete reactions of rhenium pentacarbonyl bromide or chloride with a bidentate ligand,5 but with the iodide the reaction was incomplete. Changing the reaction solvent to 1-propanol with a boiling point of 97-98 °C, 32-33 °C higher than methanol, lowers the reaction time. Four-hour reactions in 1-propanol led to complete reaction with no adverse effects from a higher reaction temperature. The reactions are robust and no attempt was made to exclude water or oxygen. The α-diimine ligands studied were 2,2’-bipyridine (bipy), 1,10-phenanthroline (phen), dimethylglyoxime (dmg) and the Schiff base from the reaction of pyridine-2-carboxaldehyde and valine methyl ester×HCl (pyca-Val-OMe); these ligands have been exhaustively studied for chloride and bromide derivatives.6
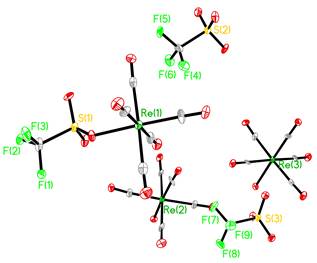
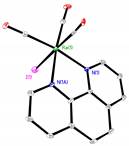
Although the synthesis of 1 is well-known, its structure had not been reported. We analyzed crystals from the preparation of 1 and discovered that the structure was significantly more complicated than expected. The structure, shown in Fig. 1, has the stoichiometry of one equivalent of 1 and two equivalents of [Re(CO)5(H2O)][OTf]. The hydrolysis of 1 during routine synthesis and crystal growing was a surprise. But its appearance is a reflection of the fact that the triflate ion is a good leaving group and a small presence of water will replace it. The discovery that instead of 1 we were dealing with 1×[[Re(CO)5(H2O)][OTf]]2 did not affect the reaction chemistry. Each of the rhenium species reacted with incoming iodide in subsequent reactions to make 2.
Fig. 1a) Molecular structure of 1×[[Re(CO)5(H2O)][OTf]]2. Hydrogen atoms have been omitted for clarity. b) Molecular structure of 2.
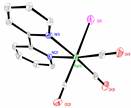
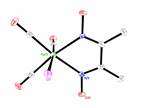
The structures of compounds 3-6 were all solved (Fig. 2). Each mimics a similar chloride or bromide structure. The NMR and other characterizations, including elemental analysis were all as expected.
Fig. 3 Molecular structures of compounds 3-6 (left to right). Hydrogen atoms have been omitted for clarity.
We are currently looking at making compounds with some water solubility to examine possible biological applications.
1. Forster, D.; Dekleva, T. W. J. Chem. Educ. 1986, 63, 204.
2. Ziessman, H. A.; O'Malley, J. P.; Thrall, J. H.; Fahey, F. H. Nuclear medicine. 4th ed.; Elsevier/Mosby: Philadelphia, PA, 2014; p p.
3. Bushberg, J. T. The essential physics of medical imaging. 3rd ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, 2012; p xii, 1030 p.
4. Adams, D. M.; Ruff, P. W.; Russell, D. R. J. Chem. Soc., Faraday Trans. 1991, 87, 1831-6.
5. Herrick, R. S.; Ziegler, C. J.; Sripothongnak, S.; Barone, N.; Costa, R.; Cupelo, W.; Gambella, A. J. Organomet. Chem. 2009, 694, 3929-3934.
6. Herrick, R. S.; Wrona, I.; McMicken, N.; Jones, G.; Ziegler, C. J.; Shaw, J. J. Organomet. Chem. 2004, 689, 4848-4855.