Reports: DNI752345-DNI7: Elucidating the Structure and Properties of Model Bottlebrush Polymers
Rafael Verduzco, PhD, Rice University
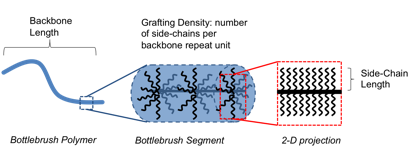
Figure 1. Schematic for a bottlebrush polymer.
We prepared bottlebrushes with temperature responsive poly(N-isopropylacrylamide) (PNIPAAM) side-chains. PNIPAAM is water soluble at room temperature but becomes water insoluble at elevated temperatures, above 32 degrees C. PNIPAAM has also been shown to reduce surface fouling, and therefore PNIPAAM bottlebrush is a potential coating or additive for the preparation of antifouling films.
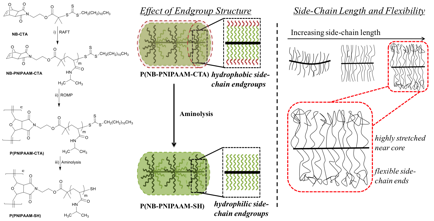
Figure 2. Synthetic scheme for the preparation of bottlebrushes with PNIPAAM side-chains and schematic for the role of side-chain endgroup and length.
PNIPAAM bottlebrushes were synthesized by a combination of controlled radical polymerization and ring opening metathesis polymerization, as shown in Figure 2. After preparation of the bottlebrushes, side-chains contained a hydrophobic endgroup that resulted in poor water solubility of the bottlebrushes. This endgroup was removed by aminolysis to give fully water soluble bottlebrushes.
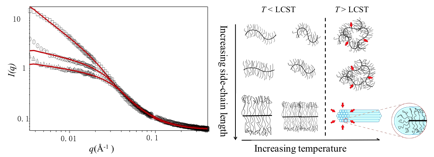
Figure 3. Small-angle neutron scattering analysis (left) and schematic phase behavior of PNIPAAM bottlebrush polymers.
Next, the PNIPAAM bottlebrushes were analyzed by small-angle neutron scattering (SANS) to determine conformational changes with temperature. SANS demonstrated that side-chains collapsed with increasing temperature, and the lower critical solution temperature was influenced by side-chain length and endgroup. In the case of the longest side-chains studied (9 kg/mol) the formation of a lyotropic liquid crystal phase was observed. Films of PNIPAAM bottlebrush polymers are being explored for antifouling coatings (see Figure 4).
Figure 4. Crosslinked PNIPAAM bottlebrush film at room temperature (left) and above 32 degrees C (right).
As an alternative to using preparing films comprised of bottlebrushes, we explored the behavior of bottlebrush polymers as additives. We hypothesized that when blended with linear polymers, bottlebrush polymers would spontaneously segregate to interfaces due to entropic interactions. To explore this, we studied athermal blends of polystyrene (PS) bottlebrush polymers blended with linear deuterated PS (dPS). Enthalpic interactions between PS and dPS are very weak, and thus this is approximately an athermal system where entropic interactions will be most important.
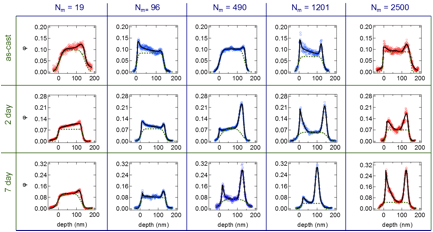
Figure 5. Secondary ion mass spectroscopy (SIMS) analysis of PS bottlebrush/linear dPS blend films. All films have 10 wt % PS bottlebrush added.
Taking advantage of the contrast between PS and dPS, films could be depth profiled by secondary ion mass spectroscopy. These measurements revealed clear evidence for segregation of the PS bottlebrushes to the top and bottom film surface. Strong segregation was seen only above a critical dPS molecular weight (roughly 5 kg/mol). Quantitative analysis of interfacial excess demonstrated that PS bottlebrushes had a slight preference for the bottom film-substrate interface over the film-air interface.
Figure 6. Schematic for the segregation of PS bottlebrushes when blended with linear dPS of a low (top) and high (bottom) molecular weight. Entropically-driven demixing, more significant at higher dPS molecular weights, drives PS bottlebrush segregation.
In related work, we synthesized bottlebrush polymers with poly(dimethyl siloxane) (PDMS) side chains. PDMS is a widely used, low cost elastomeric material that has low surface energy and is biocompatible. To date, only very short branched PDMS has been explored, but through ring-opening metathesis polymerization we are able to synthesize PDMS bottlebrushes with over 70 PDMS side-chains. The resulting PDMS bottlebrushes are liquids, even at molecular weights over 375 kg/mol.
Figure 7. Schematic for the synthesis of mixed bottlebrush copolymers with PDMS and PS side-chains.
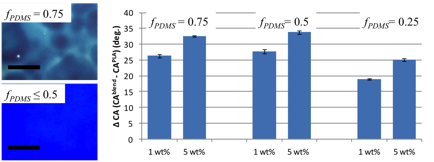
To explore the properties of PDMS bottlebrush polymers as coatings, PDMS side-chains were co-polymerized with poly(lactic acid) (PLA), which has a higher glass transition temperature. In contrast to PDMS bottlebrushes, PDMS/PLA mixed bottlebrush polymers are solid at room temperature and can be deposited on a surface through spin casting. We explored PDMS/PLA bottlebrush polymers as additives that spontaneously segregate to the film surface. Mixed bottlebrushes with 50 wt % PDMS or lower formed uniform blends with PLA, and the addition of just 1 wt % PDMS/PLA bottlebrushes produced up to 35 degree change in the water contact angle.
Figure 8: Optical microscopy and water contact angle measurements of mixed PDMS/PLA bottlebrush polymer films blended with linear PLA. Scale bar = 100 micrometers. The plot shows the change in the water contact angle in films with PDMS/PLA bottlebrush additives relative to pure PLA.
Figure 9. Verduzco lab group picture, July 2014.
This project has produced five publications to date and one more currently under review. Four graduate students and three undergraduate students have contributed to this project. Xianyu Li led the efforts with PNIPAAM bottlebrush polymers and bottlebrush polymer blends. Xianyu recently graduated with her PhD and is currently employed as a research scientist at Envia, Inc. Stacy Pesek is a fifth-year graduate student in the group and is focusing on the development of PDMS bottlebrushes along with PLA and PEG bottlebrush polymers. Luqing Qi is a third year graduate student looking at the structure and properties of PNIPAAM additives at the oil-water interface. Qiqi Xiang was a Rice undergraduate student and is now a master student studying the film properties of bottlebrush polymer additives. Will Kasper is a current undergraduate student working with Stacy Pesek on the synthesis and characterization of PDMS bottlebruhses. This past summer Ralph Cox, a high school student, worked on film characterization as part of an NSF-funded research experience for teachers (RET) program. Ralph studied the properties of PDMS bottlebrushes as additives through water contact angle measurements. Finally, the work with bottlebrush blends has led to a new collaboration with Dr. Gila Stein's group at the University of Houston.
Figure 10. Dr. Rafael Verduzco and Dr. Xianyu Li.. Xianyu Li successfully defended her Ph. D. thesis entitled "Structure and Applications of Bottlebrush Polymers" in May 2014.