Reports: ND1053490-ND10: Metal-Organic Framework Catalyst for Organic Compound Conversion
Nianqiang Wu, PhD, West Virginia University
The project aims to develop a synthetic approach to tune the band gap of the metal-organic frameworks (MOFs) for catalytic organic transformation reactions. The specific tasks laid out in the project are:
(1) Synthesize a series of MOFs with different side groups substituted on the organic linker via a one-step method or a post-synthesis modification process.
(2) Identify the correlation of the optical band-gap and electronic structure of the organic framework with the substituted side groups.
(3) Investigate the photocatalytic activity of MOFs toward the aerobic organic transformation.
2. Significant Results
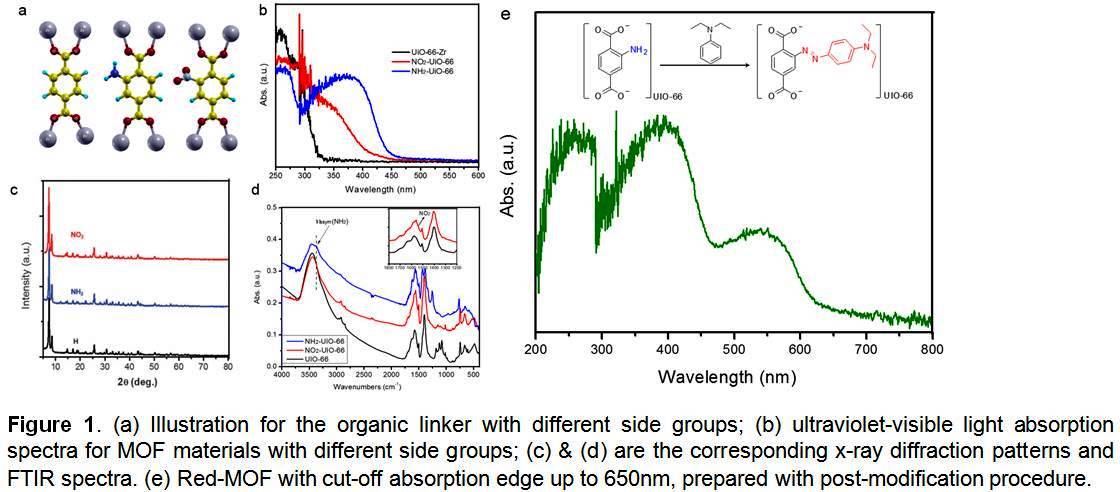
MOFs are an attractive photocatalyst because of their high surface area and easily modified organic structure. However, the band gaps of MOFs fall into the ultraviolet (UV) light region. Most photoactive MOFs have a band gap larger than 3.4 eV. We have designed and synthesized several Zr-based MOF materials with different side groups to extend UiO-66-Zr into visible light region. Figure 1a shows the structure of the organic linkers with different side groups (H, NO2 and NH2). The synthesis of the MOF materials was confirmed with X-ray diffraction and FTIR characterization (Fig. 1c&d). The presence of side groups did not change the crystallography of the parent MOF material.
Figure 1b shows the ultraviolet-visible absorption spectra for the as-synthesized MOF materials. Parent MOF UiO-66-Zr shows deep-UV light absorption, which has a cut-off edge at about 325 nm, corresponding to a band gap of 3.76 eV. Both NO2 and NH2 side groups can extend the light absorption region. NO2-UiO-66 showed a yellow color with a band gap of 2.93 eV. NH2 modification exhibited the larger positive effect on the light absorption, shifting the cut-off edge up to 450 nm with a band gap of 2.75 eV. Recently, we post-modified NH2-UiO-66 and grafted a dye group on the benzene ring, which resulted in a light absorption up to 600 nm (Fig. 1e). This means that linker modification indeed altered the electronic band structure of MOF materials, which has offered an effective way to tune the band gap of this kind of organic-inorganic hybrid materials.
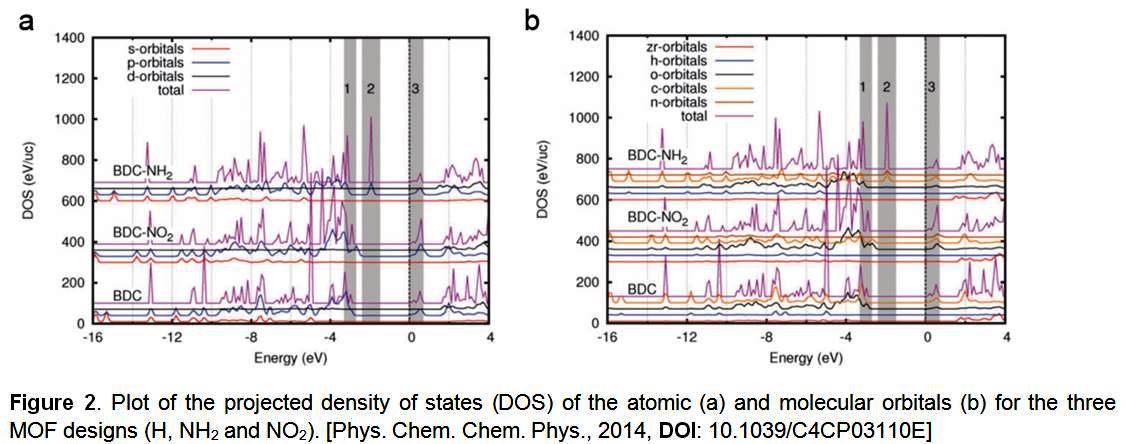
To understand the correlation of the electronic band structure of MOF materials with the functional side groups, the density-function theory (DFT) calculation has been performed in collaboration with Dr. Terence Musho to investigate the density of states and the origin of the modulation.
In NH2-UiO-66-Zr, a significant peak in the density of states between -3 eV and -2.5 eV (Fig. 2a) can be observed, which originates from the donor states of p-orbitals as would be expected from the attached nitrogen atom that has a 2p valence state (gray region 2, Fig. 2a). Both the carbon atoms and the nitrogen atoms are contributing to the states (gray region 2, Fig. 2b). The H-N donor site results in a decrease in the band gap of NH2-UiO-66.
However, for the NO2-UiO-66, the decrease in the band gap is realized by addition of the states near the valence band maximum (gray region 1, Fig. 2). The aromatic ring when joined with NO2 will be slightly rotated due to the directional nature of the sp2 hybridized bond between the functional group and the aromatic carbons. The functional group in this configuration serves as an acceptor since the nature of the nitrogen-oxygen bond is not fully satisfied and is able to accept p-orbital electrons from the sp2 hybridized carbon atoms, resulting in a slight modification of the p-orbitals. Contribution of states in the valence band maximum originates from both carbon atoms and oxygen on NO2 group (Fig. 2b), which is not present in NH2 group. Therefore, the oxygen at the end of the functional group interacts with aromatic carbons by rotating out-of-plane of the aromatic ring. As a result, the oxygen accepts electrons from the carbon atoms, that leads to a shift of carbon states at the valence maximum. A combination of nitrogen and oxygen interacting with the carbon results in the decrease of the band gap.
This finding offers evidence that the band gap can be further modulated if donor states from additional or improved functionalization can be localized near the sp2 carbon atoms or the aromatic ring. It is suggested that large functional groups even though they may have donor contributions may prove to be less significant if they are not localized around the aromatic carbon. This means that the functional group should focus on modifying the absorption properties of the sp2 carbon and less the functional group itself.
3. Impacts of Research
The ACS Petroleum Research Funds have helped the PI with exploring MOF catalysts, an exciting new area. The research will lay the foundation for my future success in this emerging area. The outcome of project promotes the development of green chemistry techniques to reduce the ecological impact of chemical processes. In addition, two graduate students and one undergraduate student are involved into this project. The interdisciplinary nature of this project drives students to get trained in materials science, electrochemistry and organic chemistry. The research has stimulated students’ interests in chemistry, engineering and physical science.